Condensed Matter Theory
Computer codes to calculate electronic structure and total energies for systems that can be modeled using periodic cells are now standard commodities. These codes can be based on muffin tin atomic spheres usually implemented as the full potential linearized augmented plane wave (FLAPW) method (Wien, Exciting) or they can use pseudoptentials with a plane wave expansions for the electronic states (CaSTEP, VASP)
We have applied these codes to a various problems in Materials Science
Strength of Materials
with JR. Alvarez, graduate student; J.S Braithwaite postdoc
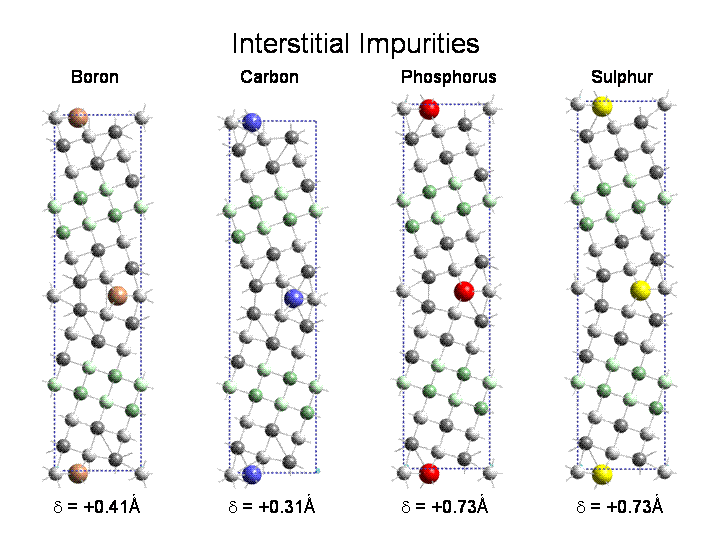
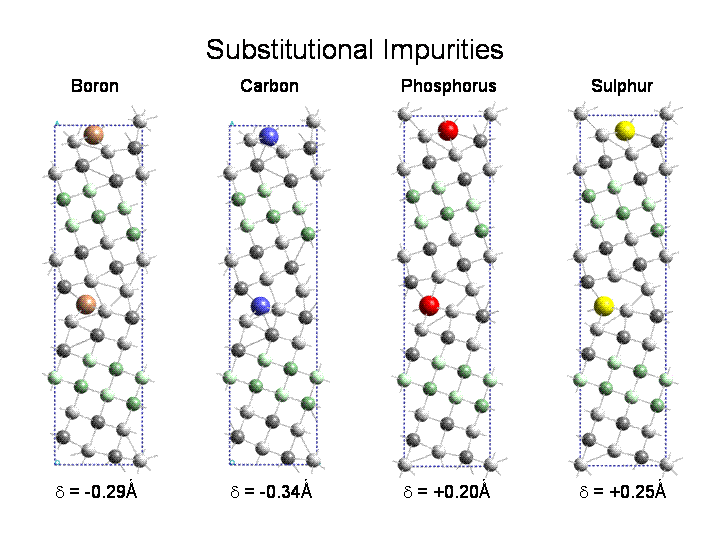
Impurities segregating to grain boundaries in metals can dramatically change the mechanical properties of the material. In some cases (B in Ni3Al, C in steel) there is a strengthening of the material, in other cases (Bi in Cu) the introduction of the impurity leads to embrittlement. In collaboration with Prof. David Williams, Department of Materials Science, Lehigh University, we applied electron energy loss spectroscopy to the study of electronic structure changes due to impurities at grain boundaries. We also investigated B in boundaries in Ni3Al and B,C,P,S, as both substitutional and interstitial impurities in (210) tilt boundaries in Fe. We found that interstitial sites are preferred over substitutional sites. The calculations also showed that B led to cohesive enhancement , while P, S led to strong embrittlement and C gave weak embrittlement except when it was present as a thin cementite layer (even 1 monolayer thick) at the boundary


References
“Grain Boundary Impurities in Iron”, J.S. Braithwaite and P. Rez, Acta Materialia, 53, 2005, 2715-2726.
"Chemistry and Bonding Changes Associated with the Segregation of Bi to Grain Boundaries in Cu", V.J. Keast, J. Bruley, P. Rez, J.M. Maclaren and D.B. Williams, Acta Met., 1998, 46, 481-49.
"Calculation of Electronic Properties of Boundaries in Ni3Al", J.R. Alvarez and P. Rez, Acta Materiala, 2001, 49, 795-802.
Li Battery Materials
Collaboration with the group of Prof. Brent Fultz at Caltech
Li based batteries are the power supply for portable electronic devices. Limitations in batteries make electric cars impractical and constrain the performance of hybrid vehicles. New and better battery materials would revolutionize tranportation.
What are the essential features of a battery?
(1)An anode which can take up large quantities of a light transportable element (Li ) in neutral form.
(2)A cathode which can take up large quantities of the same element in ionic form. Neither the anode nor the cathode can change phase when the transportable element goes in and out. They must also have as low a density as possible and be reasonable electric conductors.
(3)An electrolyte through which the transportable element moves between anode and cathode and the other way round.
Graduate student M.P. Kocher, postdoc J.S. Braithwaite
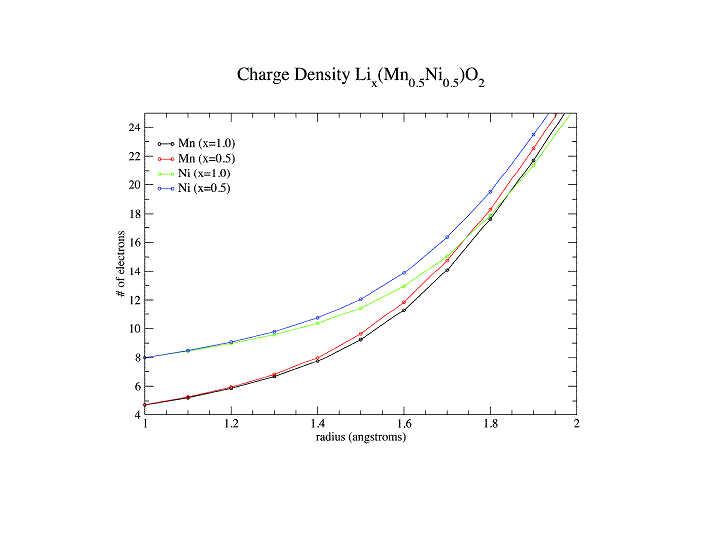
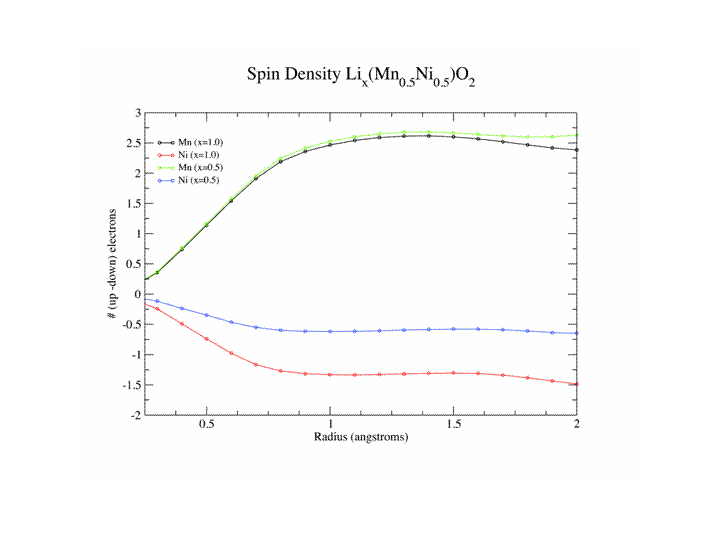
We have used a combination of electron energy loss spectroscopy and first principles DFT calculations to understand the electronic structure changes in both anode materials such as graphite and cathodes materials such as LixCoO2 LixNiMn)0.5O2, Lix(NiMnCo)0.333O2 and LiFePO4. In anode materials the Li is neutral not ionized (otherwise there wouldn’t be a potential difference between cathode and anode !)
Contrary to popular belief we have shown that the cathode accommodates the insertion and removal of Li not by having the transition element ion change its charge state but by a change in bonding of the oxygen. For the phosphate system, proposed as an alternative to the cobaltate to overcome problems of heating, there is a change in the charge state of Fe going from LiFePO4 to FePO4.
References
"Electron energy-loss spectrometry on lithiated graphite”, A. Hightower, C.C. Ahn, B. Fultz and P. Rez, Appl. Phys. Lett, 2000, 77, 238-240.
“Electronic structure of chemically-delithiated LiCoO2 studied by electron energy loss spectrometry”, J. Graetz,, A. Hightower, C.C. Ahn, R. Yazami, P. Rez and B. Fultz., J. Phys. Chem., 2002, B 106, 1286-1289.
“Local electronic structure of layered LixNi 0.5Mn 0.5O2 and Li xNi1/3Mn1/3Co1/3O2 “, S. Miao, M. Kocher, P. Rez, B. Fultz, Y. Ozama, R Yazami and C.C. Ahn , J. Phys. Chem. B 109, 2005, 23473-23479.
“Local electronic structure of olivine phases of LixFePO4”, S. Miao, M. Kocher, P. Rez, B. Fultz, R Yachimi, C.C. Ahn, J. Phys. Chem. A 111, (2007), 4242-4247.
Biominerals
We have been using VASP to investigate possible structures for Amorphous Calcium Carbonate Biomineralization