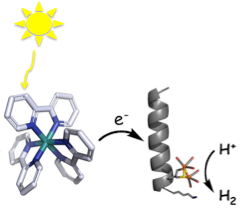
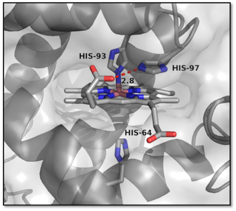
Artificial hydrogenases based on [FeFe] centers.
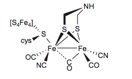
Hydrogenases catalyzes proton reduction in mild conditions, using base metals such as iron at the active site. The [FeFe] hydrogenases incorporate a small diiron organometallic complex, which is inactive in isolation, and force it into a thermodynamically unstable, but catalytically active conformation through crucial protein contacts.
The growing importance of hydrogen as possible fuel, in alternative to carbon-based, non renewable fuels, has spurred much interest in sustainable methods for hydrogen production. Unfortunately, several obstacles prevent the direct use of hydrogenases in technological applications.
We use unnatural amino acids with dithiol side chains within a protein core that can coordinate and stabilize diiron sites of bioinspired organometallic catalysts. Using this strategy we have demonstrated nascent hydrogen production activity by a small helical peptide in water at mild pH. Current work is focusing on extending this strategy to the design of complex, evolvable four-helix bundle that incorporate organometallic catalysts.
Cobalt porphyrins.
We are exploring the ability of natural porphyrin-binding proteins to catalyze hydrogen production. Generally, these proteins bind the iron form of the porphyrin, heme. However, it is possible to swap the central metal for cobalt. We found that cobalt myoglobin catalyzes hydrogen production in the presence of light and a photosensitizer, with turnover numbers approaching 300. This activity is further augmented by single-point mutations within the protein cavity.
Electron transfer proteins.
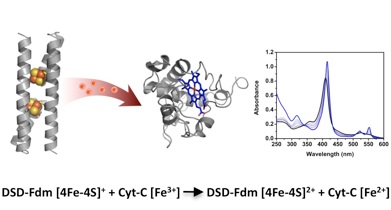
We designed a family of d 2[4Fe-4S] ferredoxin mimics, exploiting the structural repeat of coiled coils and the symmetry of the starting scaffold to modulate the distance between the two clusters. In DSD-Fdm the two clusters are positioned within a distance of 12 Å, compatible with the electronic coupling necessary for efficient electron transfer. This protein can undergo energy transfer in the presence of a photosensitizer and transfer electrons to cytochrome c, thus generating the reduced cyt c stoichiometrically.