MSE 350
Homework Set #3
Solutions
2.5.4 Calculate the attractive force between a pair of K+ and F- ions that just touch each other. Assume the ionic radius of the K+ ion to be 0.133 nm and that of the F- ion to be ).136 nm.
Solution: Using coulombs law to calculate the attractive force.
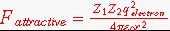
where:
Z1 = number of electron charge units on ion 1 (signed integer)
Z2 = number of electron charge units on ion 2 (signed integer)
qelectron = charge of electron, 1.60x10-19 Coulombs.
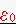 = permittivity of free space, 8.85x10-12 Coulombs2/(Nm2)
= permittivity of free space, 8.85x10-12 Coulombs2/(Nm2)
r = distance between centers of charges = sum of ionic radii
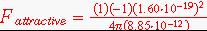
F =- 3.18x10-9 N (a positive value is also an acceptible answer)
2.56 Calculate the net potential energy of a K+F- pair using the ionic radii given in problem 2.54 and assuming n =8.
Solution: First calculate a value for b in equation 2.7.
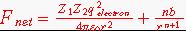
Here F net = 0 when r = sum of ionic radii.

Then calculate the net potential energy using the equation
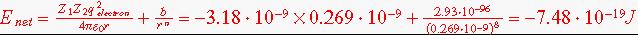
2.9.4. For each of the following compounds state whether the bonding is essentially metallic, covalent, ionic, van der Waals, or hydrogen: (If ionic and covalent bonding are involved in any of the compounds calculate the percent of ionic bonding)
a) Ni; Primarily metallic bonding with some covalent bonding of d electrons
b) ZrO2; Electronegativity factor of Zr is 1.2 and of O is 3.5.

%covalent = 27%
c)graphite; Graphite is the stable form of solid carbon it has a layered structure with the atoms in a hexagonal network covalently bonded. The bonding between layers is weaker van der Waals type bonding.
d) solid Krypton; van der Waals bonding
e) Si; covalent bonding
f) BN; Electronegativity factors B = 2.0 N=3.1
![]()
%covalent = 74%
g)SiC; Electronegativity factors Si = 1.8 C=2.5
![]()
%covalent = 88%
h)Fe2O3; Electronegativity factors Fe = 1.7 O=3.5
![]()
%covalent = 44%
i)MgO; Electronegativity factors Mg = 1.3 O=3.5
![]()
%covalent = 30%
j)W metallic
k)H2O within the molecules; Electronegativity factors H = 2.1 O=3.5
%ionic = (1-exp(-(3.5-2.1)2/4)x100 = 39%
![]()
%covalent = 61%
l)H2O between the molecules; Hydrogen bonding.