CHEM 341
PHYSICAL CHEMISTRY
EXAM 3
Name____________KEY_____________________
Do not open this exam until
told to do so. The exam consists
of 6 pages, including this one. Count
them to insure that they are all there.
Constants and
conversions:
R = 8.31 J K-1
mole-1 NA
= 6.02 x 1023
F = 96500 J V-1
mole-1 A
= 0.509 for water
Do not write in the area below
Page |
Score |
|
2 |
/16 |
|
3 |
/16 |
|
4 |
/28 |
|
5 |
/28 |
|
6 |
/12 |
|
Total |
/100 |
Conceptual Problems (each
problem is worth 4 points).
1) The anode of an electrochemical cell is always
a) The positive electrode.
b) The negative electrode.
c)
The electrode the
takes up electrons from solution.
d) The electrode the gives off electrons to the solution.
2) Given the half reaction:
Cu2+ + 2e- à Cu(s) E0 = 0.34 V.
What is the midpoint potential of the half reaction
2Cu(s) à 2Cu2+ + 4e- E0 = ?
a) 0.34 V
b)
–0.34 V
c) 0.68 V
d) –0.68 V
3) The midpoint potential of a half reaction for reduction of a compound could best be described as:
a)
The potential at
which there is an equal amount of the oxidized and reduced compound.
b) The potential at which the free energy for the reaction is zero.
c) The point at which the potential of the cell is half depleted.
d) The point at which E = ½ V.
4) When we say that oxygen binds cooperatively to hemoglobin it means that:
a) Oxygen molecules associate in solution and bind as a group.
b) One oxygen molecule does not interfere with the binding of another.
c) The dissociation constant of binding the second and subsequent oxygens is larger than the dissociation constant for binding the first oxygen.
d)
The dissociation constant of binding the second and subsequent
oxygens is smaller than the dissociation constant for binding the first oxygen
5) Gene activation sometimes requires the binding of two transcription activators to the controlling sequence of the gene in order to activate it (one factor binding alone does not turn on the gene). Which of the following is true under these conditions?
a)
This results in a binding curve that has a sharper dependence on
transcription factor concentration.
b) This results in a binding curve that has a shallower dependence on transcription factor concentration.
c) This results in a binding curve that is maximum at a specific transcription factor concentration.
d) This results in a binding curve that never saturates regardless of transcription factor concentration.
6) The molar free energy change for transporting a neutral molecule from the inside to the outside of a membrane when the concentrations of this molecule on the two sides of the membrane are the same and the voltage across the membrane is more positive on the inside is:
a) Positive
b) Negative
c)
Zero
d) Not enough information to tell
7) The molar free energy change for transporting a positive molecule from the inside to the outside of a membrane when the concentrations of this molecule on the two sides of the membrane are the same and the voltage across the membrane is more positive on the inside is:
a) Positive
b)
Negative
c) Zero
d) Not enough information to tell
8) The molar free energy change for transporting a positive molecule from the inside to the outside of a membrane when the concentration of this molecule is greater on the outside and the voltage across the membrane is more positive on the inside is:
a) Positive
b) Negative
c) Zero
d)
Not enough
information to tell
Numerical Problems
(each problem is worth 14 points). All work must be shown for credit.
9)
Consider the reaction:
Succinate + NAD+ à
Fumarate + NADH + H+
(Note that fumarate has two less H atoms than succinate.)
Please determine: E0, DG0 and KEQ for this reaction.
The relevant half reactions are (biochemical standard states are assumed):
Fumarate +2e- + 2H+ à Succinate E0 = -0.31 V
NAD+ + 2e- + H+ à NADH E0 = -0.32 V
Succinate
à Fumarate +2e- +
2H+ E0 =
0.31 V
NAD+
+ 2e- + H+ à NADH E0
= -0.32 V
Succinate
+ NAD+ à
Fumarate + NADH + H+ E0
= -0.01V
DG0 = -nFE0
= -(2)(96500 coulombs/mole) (-0.01V) [Note:
a volt is a joule/coulomb]
= 1930
J/mole
Keq
= exp(-DG0/RT) = 0.459
10) For a solution of 50 mM sodium chloride (NaCl) + 100 mM magnesium acetate (Mg(CH3COO)2), determine the average activity coefficient for the Na+/Cl- ions (g±). Both of these salts dissociate completely (Mg(CH3COO)2 dissociates to form Mg2+ and 2CH3COO-).
I = 0.5([Na+](1)2
+ [Cl-](-1)2 + [Mg2+](2)2 + [CH3COO-](-1)2)
log g± = -|z+z-|A sqrt(I) = -0.301
g± = 0.500
11) Consider the
situation outlined below in which an Enzyme, E, is only active when two
different regulators are bound to it, R1 and R2. These regulators bind with dissociation
constants, K1 and
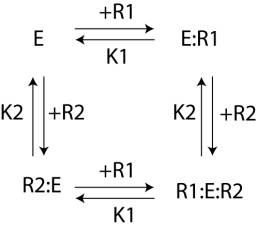
What fraction of the enzyme is in the active state when [R1] = K1 and
[R2] =
Activity = [R1:E:R2]/([E] + [E:R1]
+ [R2:E] + [R1:E:R2] =
[R1][E][R2]/(K1
[E] + [R1][E]/K1 + [R2][E]/K2 +
[R1][E][R2]/(K1
[R1][R2]/(K1
1 + [R1]/K1 + [R2]/K2 +
[R1][R2]/(K1
12) Consider the half reaction NAD+ + H+ + 2e- à NADH E0 = -0.320
Note that the E0 given is for the biological standard state. What is the E for this reaction when the pH is 5.0 but everything else is kept in the biological standard state (remember the biological standard state is pH 7.0)?
E = E0 - (RT/nF)
ln([NADH]/([NAD+][H+])
All of the chemical
concentrations are standard state and therefore appear as 1.0 in the equation
except for H+. In this case
the concentration relative to the biological standard state is 10-5/10-7
= 102 since pH 7 is the biological standard state, so the equation
becomes:
E = -0.320 - {(8.314 J/K
mole)(298K)/((2)(96500 Col/mole))} ln((1)/((1)(102)))
= -0.261V
Quasi Real World
Problem (12 points)
13) Many cells in your body have an ion pump in their membranes that pumps out three sodium ions (Na+) and pumps in two potassium ions (K+). Note that the two processes are coupled: every time three sodium ions are pumped out, two potassium ions are pumped in. Normally, the external concentration of Na+ is 145 mM and K+ is 4 mM while the internal concentration of Na+ is 12 mM and K+ is 150 mM. If the membrane potential is 150 mV (the voltage is more positive on the outside, negative on the inside) what is the molar free energy for this reaction at T = 310K?
3Na+In + 2K+Out à 3Na+out + 2K+In
Hint: z in the expression zFV is the NET charge transferred across the membrane.
DG = RTln([Na+out]3[K+In]2/([Na+in]3[K+out]2)
+ zFV
= (8.314 J/K mole)(310 K) ln ((0.145)3(0.150)2/((0.012)3(0.004)2))
+ (1)(96500 Col/mole)(0.150V)
= 37.9 kJ/mole + 14.5 kJ/mole =
52.4 kJ/mole