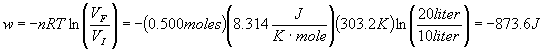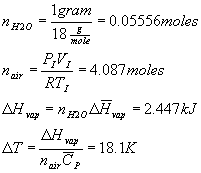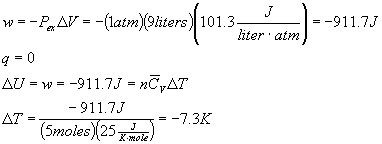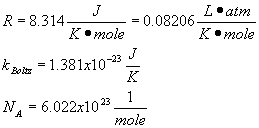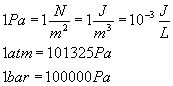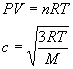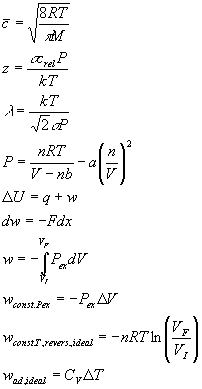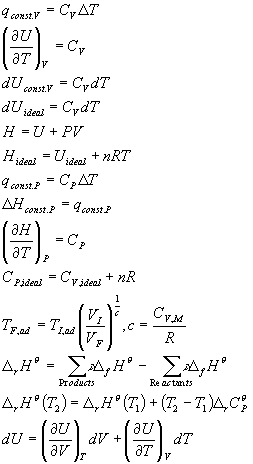CHEM 341
PHYSICAL CHEMISTRY
EXAM 1
Name____________KEY_____________________
ID#_______________________________________
Do not open this exam until told to do so
. The exam consists of 6 pages, including this one. Count them to insure that they are all there. The last page of the exam is a list of constants and equations. No additional notes are allowed. You should only have your exam, writing implements and a calculator on your desk.
Do not write in the area below
|
Page |
Score |
|
2 |
/ 24 |
|
3 |
/ 38 |
|
4 |
/ 19 |
|
5 |
/ 19 |
|
Total |
/100 |
Conceptual Problems (each problem is worth 6 points). You must give a brief explanation of your answer for full credit (a sentence or an appropriate mathematical expression).
- Heat is transferred to a system. Will the internal energy increase more if the system is at constant volume or at constant pressure? Explain. Assume that the constant pressure system is surrounded by an environment at 1 atm.
The internal energy will increase more if the system is at constant volume because at constant pressure, some of the energy input as heat goes into work done on the surroundings instead of all going into the kinetic energy of the gas molecules.
- For the isothermal, reversible compression of an ideal gas, will the heat (q) be positive, negative or zero? Explain.
Since the expansion is isothermal and of an ideal gas, the change in internal energy is zero. This means q = -w and for a compression, w is positive. Therefore q must be negative.
- Burning gasoline inside a closed, adiabatic system at constant volume will cause the internal energy to increase, decrease or stay the same? Explain.
The change in internal energy of any closed adiabatic system at constant volume is zero (assuming that only PV work is possible).
- One mole of an ideal gas is taken from a state in which P = 1atm, T= 25 C, and V = 1 liter to a state in which P = 1.2 atm, T = 192 C and V= 1.3 liters. Which changes more, the internal energy or the enthalpy? Explain.
The change in internal energy will just be given by CV times the change in temperature. The change in enthalpy will be given by CP times the change in temperature. Therefore, the change in enthalpy will be greater since for an ideal gas CP = CV + nR.
Numerical Problems (each problem is worth 19 points). All work must be shown for credit.
- 0.500 moles of an ideal gas is expanded isothermally and reversibly from 10.0 liters to 20.0 liters at a temperature of 30.0 C. Determine q, w, DU and DH for the process. Express all energies in Joules.
Since the system is isothermal and an ideal gas, we immediately can write down that
D
H = DU = 0. This of course means that q = -w. For an isothermal expansion,
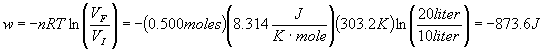
Therefore w = -873.6 J and q = 873.6 J.
- For the reaction, H2 + 1/2 O2 à
H2O, (all in the gas phase) the standard enthalpy of reaction at 25.0 C is -241.8 kJ/mole (-241.8 kJ per mole of water formed). If the molar heat capacities at constant pressure of H2, O2 and H2O(gas) are 28.82, 29.36 and 33.58 J/(mole K), respectively, what would the enthalpy of reaction be at 50.0 C? Assume that the gases all behave ideally before and after the reaction occurs. State your answer for this problem to 4 significant digits.
Here the strategy is to realize that enthalpy is a state function. So we start with 50 C hydrogen and oxygen, cool them to 25 C, undergo the reaction at that temperature, then heat the H2O gas back up to 50 C.
DH
R,50 = -CP,H2 DT - (1/2)CP,O2 DT + DHR,25 + CP,H2O DT
The first two terms are for cooling one mole of H2 and half a mole of O2 from 50 to 25 C (they are negative because this is cooling and heat is leaving the system). The next term is the reaction enthalpy at 25 C given in the problem for one mole of water formed. The last term is heating the H2O from 25 to 50 C (which is positive because this requires heat from the environment). Evaluating:
DH
R,50 = -(28.82 J/K)(25 K) - (1/2)(29.36 J/K)(25 K) - 241800 J + (33.58 J/K)(25 K)
= -242000 J
So the reaction enthalpy at 50 C on a molar basis is -242.0 kJ/mole. A very small change from the reaction enthalpy at 25 C.
- 100 liters of an ideal gas at 25.0 C is placed in a closed, adiabatic container that is kept at a constant pressure of 1.00 atm. 1.00 gram of liquid water is then introduced into the container. Assuming that all of the water in the container evaporates, what would the final temperature be? Ignore the effects of the evaporated water on the number of moles of gas molecules in the container. The molar CP for the ideal gas is 33.0 J/(K mole) and the molar enthalpy of vaporization for water in this temperature range is 44.0 kJ/mole.
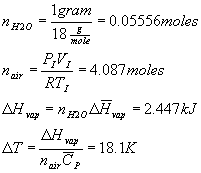
It must be cooling since energy must be taken up from the gas to evaporate the water, thus:
Tfinal = 6.9 C
Applied Problem (19 points)
8) In principle, you could build a refrigerator using an ideal gas instead of freon. (In practice, this is impractical because of the large volumes that would be necessary to do the job, but we can consider it in theory.) Consider a refrigeration cycle where 5.00 moles of an ideal gas in 10.0 liters at 25.0 C are compressed reversibly and adiabatically to 1.00 liter, then cooled to room temperature (25.0 C), then expanded back to 10.0 liters adiabatically against an external pressure of 1.00 atm. This cool gas is then run through the refrigerator to cool it down. What would the temperature of the gas entering the refrigerator be (right after the adiabatic expansion)? Assume that the molar CV for the ideal gas is 25.0 J/(K mole).
For an adiabatic expansion:
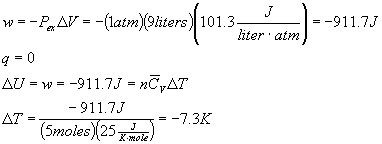
Tfinal = 17.7 C
Summary of Equations and Constants for Exam 1
Constants
Equations