![]()
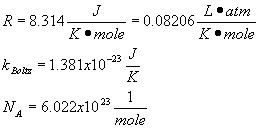
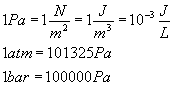
![]()
CHEM 341
PHYSICAL CHEMISTRY
EXAM 2
Name______________
KEY___________________
ID#_______________________________________
Do not open this exam until told to do so
. The exam consists of 6 pages, including this one. Count them to insure that they are all there. The last page of the exam is a list of constants and equations. No additional notes are allowed. You should only have your exam, writing implements and a calculator on your desk.
Do not write in the area below
|
Page |
Score |
|
2 |
/ 24 |
|
3 |
/ 38 |
|
4 |
/ 19 |
|
5 |
/ 19 |
|
Total |
/100 |
Conceptual Problems (the first two are 6 points each and the last is 12 points). You must give a brief explanation of your answer for full credit (a sentence or an appropriate mathematical expression).
D
G < 0 for a spontaneous process. Recall that DG = -TDSTot. The second law can be stated that DSTot > 0 for a spontaneous process. Thus DG < 0. Another way to do this is to say DSSys > q/T for a spontaneous process. At constant pressure this means that DSSys > DH/T for a spontaneous process. DG = DHSys -TDSSys and therefore must be negative for a spontaneous process.During an isothermal reversible expansion, work is done by the system and thus heat must be taken up from the surroundings to keep the temperature constant. Since the environment is losing heat, the sign of the entropy change for the environment is negative.
3) Consider burning a piece of wood inside a closed, adiabatic, constant volume container filled with oxygen. Determine whether each of the following are positive, negative, or zero and explain why.
a) The change in internal energy.
The change in internal energy is zero since q = 0 (adiabatic) and w = 0 (constant volume, no PV work).
b) The change in the entropy of the system.
Since burning wood in oxygen is spontaneous, the change in total entropy must be positive. Since the system is adiabatic (no heat taken or given to the outside), we know that the change in entropy for the surroundings is zero. This means the change in entropy inside must be positive.
c) The change in the entropy of the surroundings.
For the reasons cited in part b, the change in entropy in the surroundings is zero.
Numerical Problems (each problem is worth 19 points). All work must be shown for credit.
4) 2.00 moles of an ideal gas is expanded isothermally and reversibly from 10.0 liters to 30.0 liters at a temperature of 20.0 C. Determine q, w, DU, DH, DSSYS and DSSUR for the process. Express all energies in Joules and entropy changes in J/K.
For an isothermal ideal gas, we know that
DU = 0 because with no change in temperature there can be no change in kinetic energy. We also know that DH = 0 because DH = DU + D(PV) = DU + D(nRT) so if T does not change both terms are zero. w = -nRTln(Vf/VI) for a reversible reaction. This comes to w = -5360 J. Since DU = 0 = q + w, q = -w = 5360 J. Since the reaction is reversible, we can write DSSys = qrev/T= 5360/T = 18.27 J/K. Finally DSSur = -DSSys because the process is reversible and therefore the total entropy change must be zero.
5) 0.200 moles of an ideal gas initially at 30 C and in 1 liter is expanded to a final volume of 2 liters and cooled to a final temperature of 10 C. Assume that the molar CV = (3/2) R. What is the change in entropy of the system for this process?
This problem must be done in two steps: change the volume holding the temperature constant and then change the temperature at constant volume.
First step: isothermal expansion
D
S1 = nRln(VF/VI) = 1.15 J/KSecond step: constant volume cooling
D
S2 = nCVln(TF/TI) = -0.170 J/KD
Ssys = DS1 + DS2 = 0.980 J/K6) 10 grams (0.556 moles) of ice at 0 C is placed in 50 grams (2.777 moles) of water initially at 80 C. The ice melts completely. Assuming that the container is adiabatic and at constant pressure, calculate the final temperature of the water and the overall entropy change for the process.
We need to do this in three steps:
1) Cool the 50 g of water in the glass to 0 C:
q1 = CP
DT = (2.777 moles)(75.5 J/(K mole))(0 - 80 K) = -16800 JD
S1 = CPln(Tf/TI) = (2.777moles)(75.5 J/(K mole)) ln(273.2/(273.2+80)) = -53.84 J/K2) Melt the ice at 0 C:
q2 =
DHfus = (0.556 moles)(6008 J/mole) = 3340 JD
S2 = q2/T = 12.2 J/K3) Warm back up to final temperature:
The overall process is adiabatic at constant pressure. Since at constant pressure
DH = q, we know that the overall DH must be equal to the overall q. For an adiabatic system, the overall q is 0 so the overall enthalpy change must be zero. At each step the enthalpy change is q (all are done at constant pressure) so the sum of all the q's must be zero. Therefore, q3 = -(q1 + q2) = 13460. From this we can calculate the final temperature as273.2 + q3/CP =273.2 + 13460/(75.5J/(K mole))/(3.333 moles) = 326.7 K. Note that all the water (including the original ice) had to be warmed back up, so the number of moles was the sum of the 10 g ice and 50 g water. Now that we know the temperature, we can calculate the entropy change:
D
S3 = CPln(TF/TI) = (3.333 mole)(75.5 J/(Kmole)) ln(326.7/273.2) = 45.0 J/KFinally,
DSSYS= DS1 + DS2 + DS3 = 3.3 J/KWe can see that since the process is adiabatic, the entropy change of the surroundings is zero and therefore the entropy change of the system is the total entropy change. In agreement with our common experience, the total entropy change is positive and thus the melting of the ice is spontaneous.
Applied Problem (19 points)
7) It turns out that you can run the Carnot cycle backwards. Instead of using it as an engine to do work which is powered by the spontaneous transfer of heat from the hot source to the cold source, I can put energy (work) into the system and transfer heat from the cold source to the hot source. Thus, in the winter I could transfer heat from the cold outside to the warm inside and in the summer transfer heat from the warm inside to the cold outside.
Calculate the amount of work it takes to transfer 1 kJ of heat into a 25 C house when the outside temperature is 10 C.
The equation for the efficiency of the Carnot engine still applies, we just have to reverse the sign of the heat and work. Thus:
e
= w/q(hot) = 1 - Tcold/Thot = 0.0503.Thus, for every kJ of heat transferred into the house (q(hot)), we will need to do
0.0503 x 1 kJ or 50.3 J of work.
Big Hint: The amount of work it takes to push the cycle backwards is equal and opposite of the amount of work you get when you run the cycle forwards. Remember that the efficiency of a Carnot engine is defined as the amount of work done by the engine divided by the amount of heat that comes in from the hot source.
Summary of Equations and Constants for Exam 1
Constants
|
|
|
Equations
|
|
|
|
Summary of equations and constants for Exam 2
|
For a spontaneous reaction:
For a reversible reaction:
For an isothermal ideal gas:
In general:
At const. Pressure
At const. Volume
Carnot Engine:
For a transition:
Isothermal, ideal gas
Useful Constants For Water -- CS (specific heat capacity of ice) = 1.95 J/( K g)
Freezing point = 0 C Molecular weight = 18 For a monoatomic ideal gas:
|