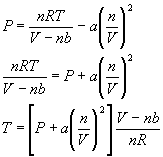
Using the Van der Waals equation and assuming a pressure of 2 atm and a volume of 10 liters what is the temperature of 0.5 moles of Argon gas (use a and b values in Table 1.5 in the book)?
We first solve equation 22a in terms of the temperature:
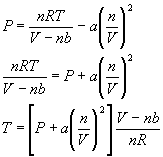
from the table, we have a = 1.345 (atm L2)/mol2 and b = 3.22 x 10-2 L/mol so plugging in gives a temperature of 243.2 K.