Lecture Set 1
Chem 341
Most of the course is on thermodynamics. What is this? It is the study of (static) states of matter, and how they differ from one another in terms of energetic quantities. The important thing to remember about thermodynamics is that it is a set of rules which applies to any object or set of objects independent of what those objects are, what they were previously, or what they will be in the future. The same rules that govern the behavior of a mole of hydrogen atoms also govern the behavior of a mole of chickens. Of course the specific energies and interactions may change depending on the substance considered, but the rules stay the same. That is the beauty of thermodynamics and it is what makes it such a tremendously powerful and useful science. It does not matter how complex the system is, it must still obey the same simple set of thermodynamic laws.
Let's say I
wanted to know how much energy my body used up each day. I could try to figure
this out by analyzing the huge number of biochemical processes occurring in my
body every second and summing the amount of energy used by each. However, this
is an essentially impossible task. Alternatively, I could simply figure out how
much energy was available from metabolizing the food I ate each day (I could
use one of the calorie counter books you can buy at the grocery store to get
these numbers), and realize that this must be the same as the energy that my
body used to stay alive, work and think (this assumes that I did not gain or
loose weight). Thermodynamics requires this absolutely. By using the first law
of thermodynamics (energy conservation) we have taken a very complex problem
and made it trivial. That is what thermodynamics is all about.
Thermodynamics really consists of a few initial assumptions and definitions and a mathematical framework in which we use these assumptions and definitions to develop useful relationships between things we can observe. The assumptions are called the laws of thermodynamics. In fact, these laws were developed empirically. They just seemed right to the people that made them up and have held up so well over the years that we now consider them to be absolute. We will deal with three laws. The so-called zeroth law is really just the definition of temperature. The first law is conservation of energy (we used this in the example above). The second law is conceptually more difficult, but is the heart of thermodynamics. One way of putting it is to say that things are more likely to be disordered than ordered (just look at my office if you want an example), and that in fact it takes energy to make things more ordered, and you can get energy by allowing things to become disordered.† As we will see, it is this law that explains why some things happen and others do not.
I want to spend a little time on this idea right now, before we dive into the details.† Think about a rubber ball.† If I drop it and it is a really good rubber ball it will pretty much bounce back up to my hand.† From physics, you all know what happened.† The potential energy of the ball in the gravitational field was converted into kinetic energy as it falls through the gravitational field.† Then when it bounces off of the floor and starts back up the kinetic energy is converted to potential energy again.† This is a classic example of the first law of thermodynamics, the conservation of energy.† But in the end, nothing changed.† Nothing really happened.† The ball went down, it came back up and, ignoring the small amount of energy lost as heat to the air and the floor, the universe was not altered by the process.† In addition, this process is time symmetric.† If you took a movie of it, you could not tell if the movie was running forwards or backwards in time.† There was no net progression from one state to another state.† Now, letís replace the rubber ball with an egg.† We drop the egg and it does not come back up.† It makes a mess on the floor.† We all know that the egg will not spontaneously assemble and jump back up into my hand.† Why?† Would that violate the first law?† No, if the egg put itself together and jumped back into my hand, the universe would simply end up where it started and therefore energy would be conserved.† But this ainít goiní to happen.† Why not?† From looking at the egg on the floor, I think you can see it has something to do with organization.† When it hit the floor, its organization changed radically, the molecules became more disordered and it would impossible for the organization to change back simply by chance.† This kind of reaction is called irreversibly.† The ability to go back is lost.† But why?† What did the egg actually lose in the process of hitting the floor?† The answer is that something called the entropy (the disorder) of the universe went up and with it time went forward (in this case you can tell whether a movie of the egg dropping is being run forwards or backwards).
The trick to thermodynamics is setting up the mathematical formalism that allows us to take the simple laws described above and to use them in the evaluation of real chemical (or physical) processes. The way physical chemists have done this is to start with a very simple system, called an ideal gas or a perfect gas, and then add to this various terms which take into account the nonideality of the real system. In other words, we start with a system which we can understand completely without any knowledge of its chemical or physical nature (in this case a bunch of noninteracting particals) and then we add the chemical and physical properties onto the system as specific parameters.
The zeroth chapter introduces the concepts of pressure and temperature and volume.† The first chapter introduces an ideal gas and goes through the definitions of the properties that we will use to describe the states of matter.
Fundamentals
What is Force?
A force is something like
gravity.† Generally speaking, it is what
causes a particle to accelerate in a particular direction.† We will normally measure force in Newtons (N).† A
What is Energy?
Energy is the capacity to do work
(heat and work are basically just forms of energy and we will discuss them
later).† We will measure energy (or work
or heat) in Joules (J), calories (cal) or electron volts (eV).† In simple terms, a joule of energy is the
amount of energy it takes to lift one kg by one meter in the earthís
gravitational field.† A calorie is the
amount of energy it takes to heat room temperature water up by 1 C.† An eV is the amount
of work it takes to move an electron (a negative charge) through 1 V of an
electric gradient.† You can see
immediately that we tend to use different units of energy to measure different
things (mechanical work, heat and electrical work in this case).
What is pressure?
Conceptually pressure is a measure of how much the surface of one substance (gas, liquid or solid) pushes against the surface of another substance. Air in an elastic balloon is a typical example. If you blow up an elastic balloon, the rubbery walls push inward on the air inside, making the air pressure inside greater than the air pressure outside.
More quantitatively, pressure is an amount of force per unit area. As
described above, force is measured in ![]() . Remember from physics
that a force (F) equals a mass (m) times an acceleration (a). Mass has units of
kg and acceleration has units of m/s2. To get the units of pressure,
we divide by area.† Putting all this
together, we have that pressure has standard units of
. Remember from physics
that a force (F) equals a mass (m) times an acceleration (a). Mass has units of
kg and acceleration has units of m/s2. To get the units of pressure,
we divide by area.† Putting all this
together, we have that pressure has standard units of ![]() . This unit of pressure we call a Pascal (Pa). We are
going to study thermodynamics in this course. Thermodynamics is intimately
related to energy. It turns out that it is very convenient to think of pressure
in terms of energy.† In terms of Joules:
. This unit of pressure we call a Pascal (Pa). We are
going to study thermodynamics in this course. Thermodynamics is intimately
related to energy. It turns out that it is very convenient to think of pressure
in terms of energy.† In terms of Joules:
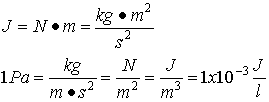
So you see that pressure can also be expressed in terms of energy per volume. Note that a liter is just a cubic decimeter. This expression will be useful later on when we do problems involving energy and volume.
So, how much is 1 Pa of pressure? Well, the pressure of our atmosphere (the pressure exerted on any surface by the air at sea level) is about 101 kPa (101,000 Pa). Since this is such an important pressure, we have a name for it and often use air pressure as a unit of pressure: 1 atmosphere (atm) = 101.325 kPa . Another common unit of pressure is 1 bar. 1 bar is often considered standard pressure and it is very close to 1 atm: 1 bar = 100 kPa. The other unit of pressure you will often encounter is millimeters of mercury. 1 mm of mercury is the pressure required to push a column of mercury up by 1 mm. A mm of mercury is also called a Torr and 1 atm is 760 Torr.
I know there are lots of units, but again, we use different units in different situations.† One of the most common problems people have in solving P-chem problems is getting the units right, so it is worth looking carefully at the description above.
What is temperature?
The next property of matter we need to define is the temperature. We will come back to a quantitative definition of temperature later, but for now we need to realize two things. First, the temperature of two things in contact with each other and isolated from the rest of the world will become equal if you wait long enough (this is the so-called zeroth law of thermodynamics). Second, temperature is essentially a measure of the amount of molecular motion (actually the energy of molecular motion, but more on that later). We tend to measure temperature relative to physical processes. One of the two most common temperature scales in chemistry is the scale derived from the boiling and freezing points of water at normal atmospheric pressure. Thus 0 C is the temperature at which water freezes and 100 C is the temperature at which it boils.
Volume and amount
The two other things that are important in defining a system are the volume of the system (usually measured in liters which are cubic decimeters or milliliters which are one thousandth of a liter or cubic centimeters) and the amount of substance. Chemists measure the amount of a substance either in terms of its weight or the number of molecules or atoms that make it up. We will be particularly concerned in thermodynamics with the number of particles and this we will measure in moles (a mole of something is 6.02 x 1023 particles (molecules) of it).
The gas laws.
What people realized early on is that in the case of many gases, pressure,
temperature, volume and amount of something are all quantitatively related to
one another. This relationship is called the ideal gas law. I think that you
can probably all see that if you take a piston and push the rod down,
decreasing the volume inside, that the pressure in the piston will increase
(assuming that there is air in the piston). Also, if you heat something up
without letting it expand, the pressure increases. Also, if you add more gas to
something without changing either the temperature or the volume, then the
pressure increases. So pressure is inversely
proportional to volume and directly proportional to temperature and amount of
gas. ![]() , where P is pressure, n is the
number of moles of substance, T is temperature and V is volume. R is the
proportionality constant and is a very special number called the gas constant.
Its value depends, of course, on the units used for the other variables and you
can find various values in your book. A more common way
of stating the gas law is
, where P is pressure, n is the
number of moles of substance, T is temperature and V is volume. R is the
proportionality constant and is a very special number called the gas constant.
Its value depends, of course, on the units used for the other variables and you
can find various values in your book. A more common way
of stating the gas law is
![]()
To explore this in more detail, we can consider how a hot air balloon works at both an overall and molecular level.
What happens if we have mixtures of gases? The beautiful thing about ideal gases is that it makes no difference what they are made of since we are assuming that they have no interactions (no specific chemical properties). This is obviously not always true, but it is an awfully good assumption for many different gases. Because it does not matter what the gas is made of, adding two or more gases in a vessel does not change the overall gas law. You just find that each individual gas contributes to the overall pressure in proportion to the amount of it in moles that you add:
![]()
This is called
This brings us to the concept of a mole fraction. Simply put, the mole fraction of gas A is the number of moles of A divided by the total number of moles of all gases in the
vessel. In other words, it is the fraction of all the gas molecules that
are A molecules. We usually write the mole fraction of
A as xA. Since the partial pressure of the
gas A is just proportional to the amount of A in
moles, then it will also be proportional to the mole fraction of A. In general, ![]() . Where Pi is the
partial pressure of the ith component and
P is the total pressure.
. Where Pi is the
partial pressure of the ith component and
P is the total pressure.
Pressure is due to collisions and we can actually derive the gas law above from the molecular theory of gases.† The point is that when a molecule hits a surface and bounces off, it exerts a force on the surface.† Lots of molecules doing this over an area of the surface exert a pressure (an total force per area).† I am not going to do it, but you could imagine that one could calculate how much force is exerted by the molecule hitting the surface and average that over lots of molecules to get the force per unit area or the pressure.
Temperature has a great deal to due with motion. In fact, it is
quantitatively related to molecular motion, or, more precisely, to molecular
kinetic energy. The kinetic energy of a molecule is given by ![]() , where m is the
mass of the molecule and v is its velocity. Since PV=nRT
and T is proportional to the velocity squared, it should come as no surprise to
learn that PV is also proportional to velocity squared (you should remember
that PV has overall units of energy). One also needs to remember that not all
molecules are going at the same velocity. Since PV is a bulk value, it must
take this distribution into account. What is found is that
, where m is the
mass of the molecule and v is its velocity. Since PV=nRT
and T is proportional to the velocity squared, it should come as no surprise to
learn that PV is also proportional to velocity squared (you should remember
that PV has overall units of energy). One also needs to remember that not all
molecules are going at the same velocity. Since PV is a bulk value, it must
take this distribution into account. What is found is that ![]() , where n is the number
of moles, M is the molecular weight and c is the root mean square velocity
(this is the velocity you get if you take all the velocities of the molecules
in your sample, square them, average them, and then take the square root).
Since PV=nRT, we can come up with an equation
relating the root mean square velocity to the temperature:
, where n is the number
of moles, M is the molecular weight and c is the root mean square velocity
(this is the velocity you get if you take all the velocities of the molecules
in your sample, square them, average them, and then take the square root).
Since PV=nRT, we can come up with an equation
relating the root mean square velocity to the temperature: ![]() . However, we
often want to know the true average speed of molecules (the sum of all the
speeds divided by the total number of molecules). This is given by
. However, we
often want to know the true average speed of molecules (the sum of all the
speeds divided by the total number of molecules). This is given by ![]() .† Which
is not very different in value from the root mean square speed, but a little.
Another interesting question is how far does the average gas molecule travel
before it runs into another molecule? This is called the mean free path and is gven by
.† Which
is not very different in value from the root mean square speed, but a little.
Another interesting question is how far does the average gas molecule travel
before it runs into another molecule? This is called the mean free path and is gven by ![]() where k is the Boltzman constant and
s is an effective collision cross
section that depends on the molecule (you look this up in a table)
where k is the Boltzman constant and
s is an effective collision cross
section that depends on the molecule (you look this up in a table)
So far, we have dealt entirely with ideal gases.† These gases take up no space and have no interactions.† While many gases behave in a nearly ideal way, none are perfectly ideal and many others are not ideal at all. In order to deal with this fact, a modified form of the ideal gas equation was developed that contains some empirical constants that take the size and interactions into account.† This is called the Van Der Waals equation:
![]()
Ok, what is going on here?† The first term of the equation is simple.† It looks just like the ideal gas equation except that V is reduced by nb.† Why?† Because real gases take up volume and so the real volume of space they have to occupy is less that the total volume.† How much less depends on how many molecules there are (n) and the volume of each molecule (b).† You can look up b in a table.† How about the other term?† This takes into account interactions.† As we will discuss later when we look at reaction kinetics, interactions between molecules tend to depend on the square of the concentration of the molecules.† (This is because the probability of two molecules being close enough together to interact depends on the probability of molecule A and molecule B both being in a small volume element.† Each of these probabilities depends on the concentration so the probability of both molecules being in the small volume element is proportional to the concentration squared.)† Since n/V (the number of molecules in the volume) is essentially a concentration, this term just says that the interactions between molecules should be proportional to the concentration of molecules squared.† This equation is still far from perfect, but it is intellectually important because it allows us to consider the effects that size and interactions should have on ideal gas behavior.†