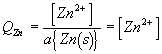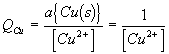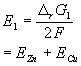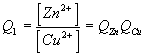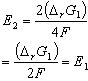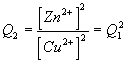Chapter 5 Notes
Ionic strength and the Debye-Huckle law
Up until now we have dealt with pretty well behaved things: ideal gases, ideal solutions, dilute ideal solutions… As long as we have kept within certain limits, such as low concentrations of solutes, we have been able to get by without have to worry too explicitly about activity coefficient and nonideality. However, there is one type of molecule which is so common in solutions (mostly water and all biochemical solutions) that we cannot ignore it, however, it is very ill-behaved indeed. These are ions. Think about what happens when you dissolve salt (NaCl) in water. The sodium and the chloride ions separate from one another giving Na+ and Cl-. The reason this reaction goes forward is because it results in an increase in entropy as the salt crystals dissolve. However, salt does not dissolve in many other liquids like oil. This is because in addition to an increase in entropy, there needs to be interactions between the ions and the solution that help to stabilize the ions in the soluble form. Water is very polar and can always align itself in such a way that their more positive hydrogens are pointing at the Cl- and their more negative oxygens are pointing at the Na+. This lowers the Gibbs free energy of the system making it possible for the anions (negative ions like Cl-) and cations (positive ions like Na+) to separate in solution.
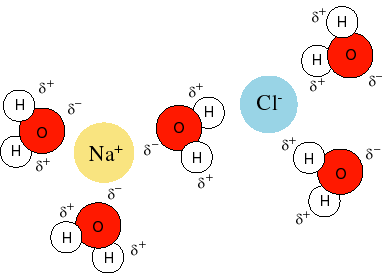
In addition to discussing ions themselves, we will also discuss reactions that either form or use up ions by adding or removing electrons from molecules in solution.
With many kinds of solvation reactions, reduction/oxidation reactions or ionization reactions we end up taking a neutral species (such as NaCl) and converting to two charge species (such as Na+ and Cl-).
NaCl(s) à Na+(aq) + Cl-(aq)
As always, we can talk about the Gibbs free energy, enthalpy and entropy of such reactions. However, if we try to break these reactions down into the Gibbs energy, enthalpy and entropy of formation of each species in the reaction, we end up defining things like the Gibbs energy of a sodium cation in solution. Yet there is no way to generate just a sodium cation in solution (that would violate charge balance). Thus, we can only determine the Gibbs energy of formation of pairs of ions. This does not cause any real physical chemistry problems, but it causes a book keeping problem: how do we write down the Gibbs energy of formation of a Sodium cation in a book so that we can use it in any reaction where sodium cations are formed?
The answer is that we come up with a sort of standard ion, H+ (really something more like H3O+) and we define the free energy of formation of all other ions relative to this one. For example, I can measure the Gibbs energy change when I dissolve HCl in water to form H+ and Cl-.
HCl(g) ß à H+(aq) + Cl-(aq) DG01
I can now just define the Gibbs energy of formation of H+ as zero and then determine the relative Gibbs energy of formation of Cl-.
DG01 = DG0F(H+) + DG0F(Cl-) - DG0F(HCl)
DG0F(H+) = 0 (definition)
DG0F(Cl-) = DG01 + DG0F(HCl)
Knowing this, I can measure the Gibbs energy of dissolving NaCl in solution forming Na+ and Cl-.
NaCl(s) ß à Na+(aq) + Cl-(aq) DG02
DG02 = DG0F(Na+) + DG0F(Cl-) - DG0F(NaCl)
DG0F(Na+) = DG02 + DG0F(NaCl) - DG0F(Cl-)
I now have a value for the Gibbs energy of formation for Cl-, so I can determine the value for the Gibbs energy of formation of Na+. I can just keep this up, relating one ion pair to another until I have Gibbs energies of formation for all individual ions relative to H+. These values I can put in a book. This works because whenever we create one ion, we always create another. Thus, we only need the relative Gibbs energy of formation for ions -- the combination of the Gibbs energies for the two ions together is all that must be absolutely correct. We will use the same trick later in the chapter to talk about the standard Gibbs energy (or enthalpy or entropy) for reactions in which an electron is transferred from one species to another, since this always involves the formation or consumption of two ions. Once you have generated this table of Gibbs energies, enthalpies and entropies of formation for ions, you use them just as you do for any chemicals.
In other words, for any reaction involving ions, you can take all the Gibbs energies (or enthalpies or entropies) of formation of the products and add them up and then just subtract the Gibbs energies of formation of the reactants, just as you have done in the past.
Ok, as I mentioned above, one of the problems with ions is that they have very strong interactions with each other over long distances. Thus, even very dilute solutions behave in a nonideal way. Thus with ions, activity coefficients tend to be rather different from one. For this reason, we much more frequently need to calculate the activity of ions then of most other chemical species (frankly, we rarely bother with neutral species). It is not unusual for the activities of ions to differ from their concentrations by nearly a factor of two. Immediately we run into the ion pair problem again. The problem is that we cannot measure Gibbs free energies or chemical potentials of individual ions, only ion pairs:
DGsoln = m+ + m-
We can expand the chemical potentials in the usual way:
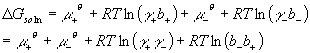
As you can see, it is not really possible to separate the activity coefficients of the cation and anion. All we get is their product. Thus we define a new ionic activity coefficient which is a geometric average of the anion and cation coefficients. For a simple monovalent ion pair like NaCl this is just:
![]()
For a salt with one or more multivalent ions like Mn+ Xm- it becomes
![]()
This is what we typically use. There are problems and ambiguities with this though. It is pretty straightforward for something like NaCl where you end up with two monovalent ions. But what about CaCl2? Here you get the average of the activity coefficient for a monovalent and a divalent ion. It gets even more confusing when there are other ions in solution as well. The thing to remember is that this is just a way of keeping track of how a particular ion pair behaves.
Next we will talk about a way of estimating what these average activity coefficients are. These are just estimates and designed only to give a rough indicator of the actual situation. The calculations are useful in that they tell you roughly how large a non-ideality you have to deal with. However, they usually do not give very precise corrections. (They are more accurate for very low concentration ions.) The method we will use is called the Debye-Huckel limiting law. The law relates the total ionic strength, which is just a measure of how ionic the solution is, and the concentration of a particular ion pair of interest, to the average activity coefficient for the ion pair. Before we can use this law, we need to learn about ionic strength.
Calculating ionic strength.
As stated above, the ionic strength of a solution is a measure of the amount of ions present. As you might guess a divalent ion (a 2+ or 2- ion, like Ca2+) does more to make the solution ionic than a monovalent ion (e.g., Na+). This must be taken into account. The other very critical thing to remember is that the ionic strength of a solution depends on the concentrations of all the ions in the solution, not just the ion pair that you are calculating the activity coefficient for. Thus, if you are calculating the average activity coefficient of dissolved CaCl2, but there is also dissolved NaCl present, the ionic strength you use has contributions from all the ions.
The formula for ionic strength is ![]() . Ionic strength is sometimes
stated as having units of molal (or molar) and other times stated as being
unitless (this is the case on your book), depending on the book you read. In
most cases, ionic strength is considered unitless, and is calculated from
concentrations relative to the standard state (1 molal). I have left the
standard molalities out of the equation below for simplicity, but in principle,
all of the concentrations are divided by 1 M. The easiest way to see how to apply
this formula is to consider a few examples. First consider 100 mM NaCl. Upon
dissolving, one obtains 100 mM Na+ and 100 mM Cl-. Thus
. Ionic strength is sometimes
stated as having units of molal (or molar) and other times stated as being
unitless (this is the case on your book), depending on the book you read. In
most cases, ionic strength is considered unitless, and is calculated from
concentrations relative to the standard state (1 molal). I have left the
standard molalities out of the equation below for simplicity, but in principle,
all of the concentrations are divided by 1 M. The easiest way to see how to apply
this formula is to consider a few examples. First consider 100 mM NaCl. Upon
dissolving, one obtains 100 mM Na+ and 100 mM Cl-. Thus ![]()
Notice that for a simple salt of two monovalent ions, the ionic strength is just the concentration of the salt. This is not true for a salt with one or more multivalent ions like MgCl2. For a 100 mM solution of this salt:
![]()
Note that the Mg cation is divalent and thus it has a big effect since the charge is squared. Also note that the chloride anion is present at twice the concentration since there are two chloride ions per molecule of salt. What is the ionic strength of a solution of 100 mM NaCl plus 100 mM of acetic acid which has been titrated with NaOH until the pH of the solution is 4.75 (the pKA of acetic acid)? When the pH equals the pKA, that means that half of the acetic acid has been converted to the conjugate base, sodium acetate. Acetic acid is uncharged and does not contribute to the ionic strength. However sodium acetate ionizes completely to form acetate anions and sodium cations. Since half was converted, there are 50 mM of each. Then we must add in the 100 mM of NaCl. So there is 50 mM acetate anion, 150 mM sodium anion, and 100 mM chloride anion:
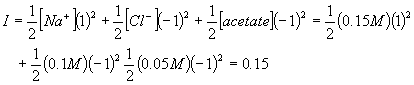
Calculating Activity Coefficients.
Now we actually will use the Debye-Huckel limiting law itself. There are
three very important things about applying the Debye-Huckel theory. First, it
only applies to ions. Molecules that are not charged have an activity
coefficient of 1.0 according to this theory (in reality, that is not true, but
their activity coefficients will be much closer to 1 than will that of an ion).
Second, the charges that appear in the equation are only those of the salt you
are calculating the activity coefficient for. Finally, all you can ever
calculate is average activity coefficient of the two ions which make up
the salt you are considering. For MgCl2, you cannot use this theory
to calculate the activity coefficient of Mg2+ separately from Cl-,
you can only calculate the geometric average of the two activities, ![]() .
.
The Debye-Huckel limiting law is ![]() where A=0.509 for water at 25 C.
(A is an empirical constant.) In the acetate/acetic acid example given above,
the sodium acetate ions would have an average activity coefficient given by
where A=0.509 for water at 25 C.
(A is an empirical constant.) In the acetate/acetic acid example given above,
the sodium acetate ions would have an average activity coefficient given by
Log g± = -|(1)(-1)|(0.509)(0.15)1/2 = - 0.197
g± = 0.635
Notice that the ionic strength is that of the whole solution, while the charges are those of the sodium acetate ions we are calculating the activity coefficient for. Remember this. It trips up many students on exams.
Electron Transfer
Now let's consider reactions in which ions are formed or consumed by the transfer of an electron (a negative charged particle) from one chemical species to another. The first thing to do is to consider the concept of a half reaction. A half reaction is not a real reaction in that it can never happen by itself. It has the form
X + e- à X-
for example. Of course, the electron must come from somewhere. That somewhere is another half reaction:
Y à Y+ + e-
In these reactions, X is getting reduced (receiving electrons) and Y is getting oxidized (giving up electrons). If we add these equations together, we get a real reaction:
X + Y à X- + Y+
An aside -- note the similarity between this formalism and the one we use for acids and bases:
X + H+ à XH+
YH à Y- + H+
Which when added gives:
X + YH à XH+ + Y-
A realistic example is:
H2O + AH à H3O+
+ A-
Reduction/oxidation reactions are really very similar to acid/base reactions, one uses electrons and the other uses protons. Neither protons nor electrons can exist in solution by themselves -- an acid needs a base to receive the proton, a reductant needs an oxidant to receive the electrons. The big difference is that water can act as both a proton acceptor and donor. It cannot act as either an electron acceptor or donor under normal circumstances (you can take electrons from water, forming hydrogen ions and oxygen gas, but this is not something that happens until you get above a fairly high ambient potential). For acids and bases we have the pKA which is the pH at which half of the acid has been converted to the conjugate base. For reduction/oxidation reactions we have the midpoint potential which is the voltage at which half of the reduced compound has been turned into the oxidized version of the same compound. I will try to point out the parallels as we go along.
Electrochemical reactions are often run in electrochemical cells (typical flashlight batteries are good examples of such cells). There is some language and nomenclature we will need to get down before we can talk about them.
Electrochemical Cell -- a system with two electrodes connected electrically which each come in contact with an electrolyte (the solution that the chemistry is occurring in). These electrodes can either be in the same solution or in two different solutions that are connected by some means (such as a salt bridge).
Galvanic Cell -- an electrochemical cell in which a spontaneous reaction generates electricity.
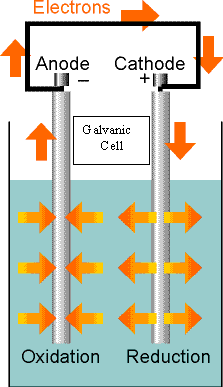
Electrolytic Cell -- an electrochemical cell in which electricity (an external voltage) is used to drive a nonspontaneous reaction.
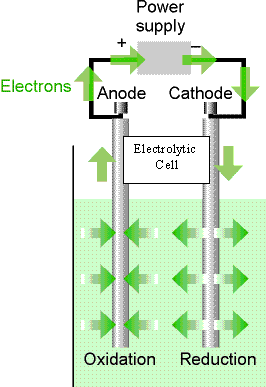
Anode -- This is the electrode that takes up electrons from the solution (do not think of it in terms of positive or negative because that changes depending on whether it is a galvanic or electrolytic cell).
Cathode -- This is the electrode that gives off electrons into the solution.
Redox couple -- The oxidized and reduced forms of a molecule, usually symbolized, for example, as Cu2+/Cu.
Electrochemical Cell Notation -- There are many ways to physically hook up cells. I am not going to worry much about this, but to do your homework you will need to know a few of the conventions about writing out the components of electrochemical cells. In the figure for the electrochemical cell below, we would write:
Zn(s)|ZnSO4(aq)||CuSO4(aq)|Cu(s)
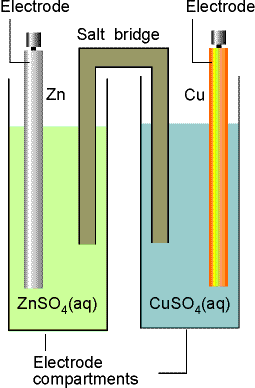
The single lines represent phase boundaries, and the double lines represent the salt bridge between electrode compartments.
As always with generation or consumption of ions, we have the problem that we can only measure processes for formation or consumption of pairs of ions. For this reason, we again define a standard half reaction and call its Gibbs free energy zero. For consistency we pick the formation of H2(gas) from H+:
2H+(aq) + 2e- à H2(g) DrG0 = 0
Just as before with the free energy of formation, we can use pairs of equations (which we can measure) to determine the relative standard reaction Gibbs energies for any other half reaction and we can write them in tables, such as the one in your book.
Finally, we need to somehow relate the electrical world (the voltage on your 1.5 volt battery in your flashlight) to the Gibbs free energy change associated with a particular electrochemical reaction. What is voltage? The units of voltage are (you guessed it) Volts. A volt is a Joule per Coulomb. What's a Coulomb? A Coulomb is 6.24x1018 charges. A Coulomb is to the world of charged particles what a mole is to the world of molecules. It is just a number, like a dozen, that we use to measure charges in. So voltage (energy per charge) tells us the amount of energy involved in moving a certain number of charges from one place to another. This is not very unlike a molar free energy. A molar Gibbs free energy is the amount of free energy it takes to convert one mole of a substance from one form or state to another.
It should come as no great surprise that the reaction free energy for an electrochemical reaction is just proportional to the Voltage it can generate.
DrG = -nFE
or for standard conditions:
DrG0 = -nFE0
Here E is the voltage (actually, it is the zero current potential -- the maximum voltage that could be produced by the system with no current flowing). The proportionality constant has two parts. Part (F, the Faraday constant) takes into account the fact that Gibbs free energy is on a per mole basis, while voltage is on a per coulomb basis and a mole is a different number than a coulomb (6.02 x 10 23 vs. 6.24 x 1018). The value of F is just 6.02 x 10 23 / 6.24 x 1018 = 9.65 x 104 C/mole. The other part of the proportionality comes from the fact that the Gibbs free energy is based on the amount of the chemical being oxidized or reduced while the voltage is based on the number of electrons that flow during the oxidation/reduction reaction. Thus n is the number of electrons involved in the oxidation or reduction reaction. For example, it is different to oxidize Fe+ to Fe2+ than it is to oxidize Cu to Cu2+. The former involves the transfer of one electron for every iron, the later requires the transfer of 2 electrons for every Cu atom. Notice also that there is a minus sign in the equation. This is because the conventions for charge movement (current) were developed before anyone knew about electrons. People just assumed that the things carrying the charges were positive. The assumption was not correct, but the convention stuck. For this reason we have to throw a negative sign into the equation.
Ok, now how do we use this equation to do something useful?
Oxidation/Reduction and half reactions.
We talked about half reactions before. In reactions that involve oxidation and reduction, it is usually the case that you can think of the reaction as occurring in two steps: one molecule gives up electrons (becomes oxidized) and another molecule picks up electrons (becomes reduced). The two parts are the half reactions. They can be dealt with mechanically (adding their free energies or multiplying their equilibrium constants to give the total reaction) in the same way as any other set of reactions can. One difference is that in writing the quotient in the free energy expression (RTlnQ), you do not include the electrons as part of Q.
Using the relationship between reaction Gibbs free energy and zero current potential, E.
![]() where the Greek letter n
in the denominator is the number of electrons in the reaction. The important
point is that in most respects E behaves just like DrG multiplied by a constant. The E’s for two coupled
reactions can be added together. If you reverse the direction of a reaction,
you just reverse the sign of E. To convert between the reaction free energy and
the zero current potential, you need to know the number of electrons involved.
You can get this from looking at the half reactions for the oxidation/reduction
reaction.
where the Greek letter n
in the denominator is the number of electrons in the reaction. The important
point is that in most respects E behaves just like DrG multiplied by a constant. The E’s for two coupled
reactions can be added together. If you reverse the direction of a reaction,
you just reverse the sign of E. To convert between the reaction free energy and
the zero current potential, you need to know the number of electrons involved.
You can get this from looking at the half reactions for the oxidation/reduction
reaction.
There are two points that differ between E and DrG:
- First, if you multiply a chemical equation by a constant, the reaction free energy multiplies with it, but the zero point potential does not. This is because the zero point potential is proportional to the reaction free energy divided by the number of electrons. When you multiply a chemical reaction by a constant value both the reaction free energy and the number of electrons change by that constant value, the ratio does not.
- Second, because of negative sign in the relationship between reaction free energy and zero current potential, a spontaneous reaction has a negative reaction free energy but a positive zero point potential.
These issues are summarized below using the example of an oxidation/reduction reaction involving Zn and Cu.
|
Reaction (or half reaction) |
DrG (see note1) |
E |
Q (see note2) |
|
Zn(s) --> Zn2+ + 2e- |
DrGZn |
|
|
|
Cu2+ + 2e- --> Cu(s) |
DrGCu |
|
|
|
Zn(s) + Cu2+ --> Zn2+ + Cu(s) |
DrG1= DrGZn+ DrGCu |
|
|
|
2Zn(s) + 2Cu2+ --> 2Zn2+ + 2Cu(s) |
DrG2= 2(DrGZn+ DrGCu) =2 DrG1 |
|
|
Note1: this reaction is spontaneous. Therefore DrG1 is negative and E1 is positive.
Note2: the activity of a pure solid is one (this is the standard state of a solid). Therefore a[Zn(s)] = 1.0 and[Cu(s)] = 1.0 and these terms do not appear in the final ratios.
The Nernst Equation:
Using the relationship between the reaction free energy and the zero current potential, one can derive a general expression for the zero current potential as a function of the standard zero current potential and the concentrations of the components in the solution:
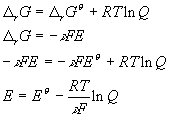
This final equation is called the Nernst Equation. You can use this equation to calculate the zero current potential given the standard potential (midpoint potential) and the actual concentrations of the reactants and products involved.
Here again, we can see a parallel between oxidation/reduction and acid/base problems. For a half reaction such as
X + e- à X-
We have
![]()
This looks rather like:
![]()
E0 in this case is the midpoint potential, the potential where half of the compound is oxidized, just as the pKA is the pH where half of the acid has been converted to the conjugate base.
pH measurements
Speaking of pH, it turns out that you actually measure pH by measuring electrochemically generated voltages for reactions involving H3O+. The measurement of pH is certainly a common thing, so it is worth having some idea how it works. In principle one can see that:
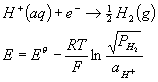
E0 is zero by definition for this reaction. If we were to set up an electrode with the pressure of hydrogen gas at the standard 1 bar:
![]()
For T=25C, we find that:
E = (59.16 mV) pH
Thus, if we had a hydrogen electrode at 25 C and measured the potential generated in a solution of some unknown pH using a solution of pH 0 (1 M H+) as a reference at the other electrode, the potential we measured would be proportional to the pH as given above:
Pt|H2(g)|1M H+||saturated KCl||X M H+|H2(g)|Pt
This represents two hydrogen electrodes (using platinum as the metal to transfer the electrons since it will not become oxidized or reduced at these potentials), one in a solution of pH 0 and the other in a solution of unknown pH. The potential generated is proportional to the pH. There is something very important to notice here. The proportionality constant is directly dependent on temperature. Thus, the pH meter will have to be recalibrated or adjusted for any temperature used (modern pH meters measure the temperature of the solution and do this calibration automatically).
Hold on, you say, you have never seen a pH meter with a hydrogen electrode. True. Gas electrodes are a pain and no one actually uses this reaction to measure pH. Instead other reactions involving H+ ions are used. Which ones depend on the electrode you buy and it is not worth our time to go through this in great detail. Most of the modern electrodes in general chemistry laboratories are actually combination electrodes where more than one reaction is going on. However, the principle is always the same and the result is the same. One gets a potential reading out which is proportional to the pH of the solution via the Nernst equation. That proportionality constant is always dependent on temperature in a linear fashion.
One sometimes wants to deal with the enthalpy and entropy of redox reactions. You could derive expressions for these in terms of E, but it makes more sense to me to convert to Gibbs energy and then use the relationships we used before, so I will not cover these new formulas in class (you may have to determine enthalpies and entropies in the homework and on the test however… again, I suggest you convert to Gibbs energy changes first and do this as we have done before).
THE BIOPHYSICS OF MEMBRANES
The book really only devotes about a page to this problem, but it is one of the most important applications of physical chemistry to biology that there is and one of the ones you will almost certainly use if you go on in the field. So, I will make up for the books omission. Lucky you.
Did you know that most of your resting energy is spent maintaining ion gradients of sodium and potassium across the cell membranes of your body? In fact, there are few aspects of energy transduction and signal transduction that do not involve membranes and chemical or electrical gradients across membranes. Production of chemical energy by respiration or photosynthesis, communication between the inside and outside of the cell, neural response, vision, muscular contraction: these are a few examples of biochemical events that depend directly on changes in chemical gradients across cell membranes and on the thermodynamics of energy storage by gradient formation.
There are two pretty simple concepts involved (well, they sound simple anyway). First, molecules like to be where they aren't. This is essentially entropy and the second law of thermodynamics. So if there are more molecules on one side of the membrane than another, there is potential energy stored in that imbalance which can be used to drive other chemical reactions (think chemical potentials and osmotic pressure). Second, if there is a net charge difference between two sides of the membrane, again this represents a potential energy source (transporting an ion across a membrane can result in a charge imbalance).
OK, lets make this a bit more quantitative. Consider the following simple chemical "reaction" which involves the movement of a molecule A across the membrane. For the moment, we will assume that A is not charged:
![]()
What is the free energy for this reaction? Well, it's just the normal thing:
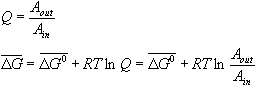
I have been very careful to draw the bars over the free energy symbols to
indicate that these are molar free energies we are talking about here. In other
words, this is the energy for transferring a mole of A from one side of the
membrane to the other. The confusing point about this fact is that we are
transferring this mole of A from one side to the other without changing the
concentration of A on either side. In other words, we are supposing that
there is an infinite volume of solution on either side of the membrane.
Obviously this is not true (try to get a mole of anything in or out of a single
cell), but we report free energy on a per mole basis anyway, even though we may
only be considering the transfer of the first molecule of A in our calculation.
You could look at it this way, we always pretend we are performing the
measurement on a mole of cells or membrane vesicles each of which is
transferring one molecule of A. What is ![]() ? Well as always we
can let the system come to equilibrium, at which point
? Well as always we
can let the system come to equilibrium, at which point ![]() is always zero, and
solve for
is always zero, and
solve for ![]() . Well, for this particular problem, I
think you can see that at equilibrium Aout =Ain and so
. Well, for this particular problem, I
think you can see that at equilibrium Aout =Ain and so ![]() must be zero. We can
therefore write:
must be zero. We can
therefore write:
![]()
Notice that the standard molar free energy for this reaction (the free energy when all of the components in the reaction have a concentration of 1M) is always zero. Compare this expression to the familiar expression:
![]()
You can see that D H = 0 and that ![]() .
In other words, since there is no bond breaking or making and no charge
interactions (we will add these later) the free energy for the reaction is
entirely given by entropy.
.
In other words, since there is no bond breaking or making and no charge
interactions (we will add these later) the free energy for the reaction is
entirely given by entropy.
Now, it is very often the case that chemical systems are more complicated than this simple one. It is often easier to break up the expression for the molar free energy into parts:
![]()
These terms are closely related to the chemical potential we spoke of earlier:
![]()
here ![]() is the standard chemical potential of
A (the potential of 1M A). The molar free energy for transferring A across the
membrane becomes just the difference between the chemical potentials on the two
sides of the membrane (the standard chemical potentials always cancel as long
as no chemical reactions occur in the process of the transfer). In general one
can write for the coupled transfer of a group of compounds across the membrane
that:
is the standard chemical potential of
A (the potential of 1M A). The molar free energy for transferring A across the
membrane becomes just the difference between the chemical potentials on the two
sides of the membrane (the standard chemical potentials always cancel as long
as no chemical reactions occur in the process of the transfer). In general one
can write for the coupled transfer of a group of compounds across the membrane
that:
![]()
Here i represents all of the different types of molecules whose transfer from one side of the membrane to the other is coupled. This last word, coupled, is extremely important and the example below should illustrate what this means.
Let’s consider the hypothetical transfer of glucose from the outside to the inside of a membrane coupled to transfer of urea from the inside to the outside. By coupled, I mean simply that a glucose molecule can only go in when a urea molecule comes out. That is to say that the mechanism of the transport requires that every time a glucose goes in one direction, urea goes in the other. There are many examples of such antiporters in biology. They are very useful, since the energy gained by allowing one molecule to travel from high concentration to low concentration can be used directly to pump another molecule from an area of low concentration to an area of high concentration. (By the way, this glucose/urea antiporter is a totally artificial example which does not exist in biology as far as I am aware. We will consider much more realistic examples later when we can deal with ions which are the usual energy coupling molecules involved in transport.) Since the two reactions are coupled, I must consider the total free energy of the two transfers together in order to determine whether the reaction will be spontaneous or not. Consider first what would happen if there was 1 mM of both glucose and urea outside and 10 mM of both compounds inside. Our reaction is
![]()
and the change in chemical potentials is given by
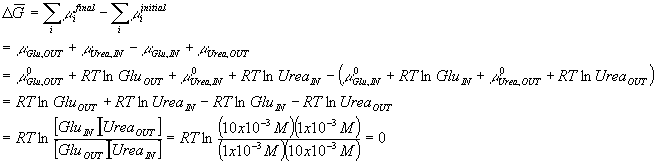
So, in this case the free energy for cotransport of glucose inside and urea outside the membrane is zero. I think that you can see that if the concentration of glucose outside had been 10 mM, the net transfer of glucose in and urea out would have been energetically favorable:
![]()
Notice that I converted between natural logs and log base 10 here. The
expression RTln(x) is conveniently evaluated for room temperature in terms of
the free energy in electron volts (just another unit of free energy – like
J/mole – which is used extensively in membrane energetics, see below) for a
ten-fold change in concentration between the two sides as 58.6 meV (58.6
millielectron volts or 0.0586 eV). Thus, 58.6 log(x) gives the value in
millielectron volts of the free energy available when the ratio of the
concentrations between the two sides of the membrane is x. Note, this is
only good at room temperature – at other temperatures you must go back to the
RTln(x) equation and evaluate that.
Now, if the glucose transport and the urea transport had not been coupled, then in order to determine if glucose would spontaneously transfer across the membrane or if urea would spontaneously transfer across the membrane I would separately have considered the two reactions. In that case for 1 mM of both compounds outside and 10 mM of both inside, we would have seen spontaneous transfer of both molecules in the outward direction with a free energy of –58.6 meV for each transfer. To show that this is true, you need only to do the same problem we did above but simply omit one of the compounds (glucose or urea) and see what free energy for transfer you get.
The balance of concentrations between molecules on the two sides of the membrane explains or quantitates the first part of the problem: the tendency of molecules to have equal concentrations on both sides. If the concentrations of all molecules are the same on both sides, the free energy for transferring one across the membrane is zero. For uncharged molecules, like glucose, that need to be transported from one side of the membrane to the other, this is how you would determine how much energy would be lost or gained in the transfer. However, very often in biochemistry we are dealing with the transfer of charged species (for example the sodium and potassium ions we talked about at the beginning). How much energy does it take to transfer a charged molecule from one side of the membrane to the other? What that depends on is both the chemical imbalance between the two sides and the charge imbalance. If there is no charge imbalance then the problem reduces to the chemical imbalance described above. Just as we saw in the case of oxidation/reduction reactions, the amount of energy it takes to transfer a charge from one side of the membrane to the other is directly proportional to the voltage difference between the two sides of the membrane.
![]()
Here z is the charge of the particle being transferred (not the number of electrons as in the Nernst equation) and F, the Faraday constant, is a proportionality constant to make the units work out (converts between coulombs of charge and moles of molecules, as we discussed previously). (Actually we often will express free energies in terms of electron volts, eV, which is just the energy required to transfer one electron across a membrane with a one volt potential difference between the two sides. In this case, F=1.0 eV/Volt). This is, of course, the same expression used in electrochemistry (do you see the similarity between transferring ions across membranes and transferring electrons between molecules in a battery?). It turns out that voltage is directly proportional to the amount of charge displaced across the membrane. The proportionality constant is called the capacitance (yes, this is the same as the capacitance in an electric circuit):
![]()
Here Q is the total charge (the number of particles times the charge per particle, z) and C is the capacitance of the membrane. For a flat membrane (actually all membranes are locally flat enough so that we can think of all membranes in this way--physicists would call this a parallel plate capacitor):
![]()
Here A is the area of the membrane, d is the thickness of the membrane and e is a proportionality constant which
physicists call the dielectric permittivity. For solution chemistry it simply
has to do with how polar or nonpolar the molecules are which are between the
two sides of the capacitor. I think you can see that in the case of water, for
example, the dielectric permittivity is high because the polar water molecules
can respond to the electric field by rotating and aligning their polar axis
along the field. This is not so true with a hydrocarbon such as the lipids in membranes.
They do not have much of a polarity to begin with and they are not really free
to realign themselves in any case. Thus a membrane has a very low dielectric
permittivity. The lowest dielectric permittivity possible is that of a vacuum.
This is called e0 and has a value of
8.854x10-12 ![]() . The units are coulombs per volt per
meter. A coulomb is a certain number of charges, much as a mole is a certain
number of molecules. As we discussed before, the physicists and chemists did
not discuss conventions with each other when they assigned these units and a
coulomb is not one mole of charge. Instead a coulomb of charge is 6.2422x1018
charges.
. The units are coulombs per volt per
meter. A coulomb is a certain number of charges, much as a mole is a certain
number of molecules. As we discussed before, the physicists and chemists did
not discuss conventions with each other when they assigned these units and a
coulomb is not one mole of charge. Instead a coulomb of charge is 6.2422x1018
charges.
It is common to talk about e as something times e0:
![]()
where K is called the dielectric constant. K = 1 for a vacuum and about 80 for water. For a membrane it is about 1 or a little more depending on the circumstances. For our purposes, we will consider it to be 1, but remember this is a point of possible error when calculating the voltage due to a particular transfer of charge across a membrane.
So, what if we have both a chemical gradient and an electric gradient (i.e. voltage) across a membrane? I hope you can see that:
![]()
Now what happens if we are dealing with only charged particles (like protons, for example) and we let protons (but no counter ions) freely go through the membrane until we reach equilibrium. For H+ the above equation would look like:
![]()
Now at equilibrium, the molar free energy is zero and we have the interesting relationship:
![]()
Which is one form of the Nernst equation (electrochemistry again, look familiar?). Notice that this is now the voltage of the inside relative to that of the outside. Voltage is always thought of in terms of movement of positive charge from one place to another. So a positive gradient of charge gives a positive voltage. (Voltage was defined before the discovery of the electron, and in those days people thought of current in terms of positive charges.)
It turns out that under normal circumstances, it takes very little transfer of charge across a membrane before the electric potential (V) that builds up due to charge displacement across the membrane is equal to the chemical potential due to the difference in the concentration of H+ on the two sides of the membrane. Thus, we normally assume that allowing the system to come to equilibrium between charge imbalance and chemical imbalance does not change the concentrations appreciably on either side (in other words, the chemical imbalance is not seriously depleted). Thus if you are told that you have a 10-fold excess of protons on one side of the membrane vs. the other and you add a protonophore (something that specifically allows transfer of protons, but nothing else, across the membrane -- a good example is nigericin -- some small organic acids will also do this) and allow protons to transfer until the chemical and electric potentials balance, then you usually assume that the ratio of proton concentrations on the two sides remains 10 to 1 (since an insignificant amount of proton transfer occurred before the equilibrium voltage developed). Note that this is not always a good assumption, but it usually is. Thus, you can use the Nernst equation to directly calculate the membrane voltage. By the way, the value of F depends on your units of energy (eV, kcals, etc.) as does R. You will need to look these up for specific applications. For room temperature, a simple rule of thumb is that a factor of ten difference between the concentration inside and outside of the membrane of a singly charged ion results in about a 58.6 mV electric potential or voltage after equilibrium is achieved (read 58.6 millivolts or 0.0586 V). As discussed above, in membrane biochemistry we often speak of free energy in electron volts. What is the difference between volts and electron volts? Volts is a unit of electric potential while electron volts is a unit of energy (usually we will use it as a unit of free energy difference between the two sides of a membrane, though it is also used in physics for other reasons). One electron volt is the amount of free energy that is required to transfer one mole of a singly charged positive ion across a membrane with an opposing electric potential of one volt. For example, the free energy change for transferring one mole of protons across a membrane with a 200 mV transmembrane potential (transferring in the direction from low to high potential) would be 200 meV. To transfer that same mole of electrons in the other direction (high to low potential) would result in a free energy change of –200 meV.
A note about conventions with membrane potentials. Unfortunately, this is one of those situations in physical chemistry where there are two arbitrary signs that come into play and there is no logical way to set your sign conventions. Both z and V can be either positive or negative and the direction of ion travel can be positive or negative. z is well defined -- it is positive for positive charges and negative for negative charges. The membrane potential is usually measured relative to the outside of the cell (with the common or negative electrode outside the cell) so that it is normally positive when it is more positive on the inside of the cell. However, we are still stuck with direction. Obviously, the free energy term, zFV, will have to flip sign if we push the molecule the opposite direction. The convention normally used (at least in neurochemistry) is that a positive ion going from the outside to the inside against a positive voltage gives a positive free energy. As far as I am concerned, this is hopeless to remember, so don't. First, I will always tell you on an exam which way the voltage is oriented (which side is positive). After that, just think about what you are doing and realize that if a charge is going towards the side that has more charges like it, that will take free energy (positive free energy), and if a charge is going towards the side with more of the opposite charges, that releases free energy (negative free energy).
Problems (Answers to problems given here).
1) a--Calculate the molar free energy required to pump one mole of a molecule A across a membrane (inside to outside) if the concentration of A on the inside is 1 mM and that on the outside is 10 mM. b--How about if the concentration outside is 1 mM and inside 10 mM. c--How about if the concentration both places is 10 mM. d-- How would it change your answers to a b and c if there was also a 10 mM concentration of molecule B on the inside and a 1 mM concentration on the outside? e-- How would the answers to a, b and c change if A had a single negative charge on it but there was no transmembrane voltage? f--How would the answers to a, b and c change if the membrane had a voltage of 100 mV across it but A was not charged? g--How would the answers to a, b and c change if there were a 100 mV voltage across the membrane and A had a single negative charge on it. For all of these problems assume that both sides have an infinite volume.
2) In theory, what is the minimum proton gradient across a membrane required for ATP production at 37 C ?
![]()
Assume that on the side of the membrane that ATP is produced ATP=5 mM, ADP=10 mM, Pi=1mM and the pH is 7.0 (remember in biology the standard state is 1M for everything except protons where a pH of 7.0 is standard). The standard free energy change associated with ATP production from ADP and phosphate is 30kJ/mole at 37 C.
3) (This one is not really very hard, but it requires that you think beyond the equations) Let's say you had a compound that could dissociate in the following way:
![]()
with a pKA of 6.0. Now let’s say we have a tube filled with chloroplasts (these are the cellular organelles in plants that do photosynthesis). By other means we have measured the total inside volume of these chloroplasts as 0.001 ml. The total volume in the tube is 1 ml. The pH outside the chloroplasts is kept constant at 8.0. We now add the weak acid described above to the tube at a concentration of 0.1 mM (assume it does not change the pH outside or inside). Note that the neutral (A) form of the weak acid can go through the membrane passively, but neither the AH+ form nor any other ion can passively diffuse through the membrane. a) assuming to begin with there is no pH or charge difference between the inside and outside of the chlorplasts, what will be the concentrations of A and AH+ inside and outside the chloroplasts? b) We now shine light on the sample and the light powered proton pump in photosynthesis starts pumping protons across the membrane from outside to inside. The photosynthetic pump is able to generate a total proton motive force (the free energy change due to both the charge and the chemical imbalance across the membrane) of about 200 meV. Assume that there is no membrane potential, only a pH gradient across the membrane (low pH inside). This is not strictly true, but is actually pretty close. What will be the concentrations of A and AH+ inside and outside the chloroplasts now?
4) Determine the final equilibrium membrane potential for a one micron
radius vesicle which initially has 1mM KCl inside and 10mM KCl outside in the
presence of valinomycin (an ionophore that allows K+ but nothing else to
transfer across the membrane) a) assuming that the influx of K+ is
too small to disturb the concentration of K+ on the inside. b)
without making that assumption and allowing the inside concentration to
change as K+ comes in. Also determine approximately how much the
final inside concentration of K+ changes in part b. We will go through part b in class – you
will not have to do this on the exam, but I expect you to understand the ideas.
Redox Reactions of Photosynthesis
Most of the information in the book centers on redox reactions in terms of electrochemical cells. This is important in chemistry, but there are not platinum electrodes in biochemical systems. However there are redox reactions that result in transfer of electrons across phase boundaries. In fact, this is a critical aspect of essentially all energy transduction in biology. This is how respiration in your mitochondria work (electron transfer across membranes is coupled to proton translocation creating proton gradients) and in the reverse process, photosynthesis (same deal but powered by light instead of reduction of oxygen to water). The book talks about linear photosynthesis in plants, but for simplicity, I am going to stick to cyclic electron transfer reactions during bacterial photosynthesis.
Essentially all of the energy we use on earth, with the exception of things like nuclear, geothermal, wind and PV solar, comes from one simple set of redox reactions. All oil came from this and all of our food comes from this. This takes place in a biological solid-state optoelectronic device called a reaction center. If I sound passionate, it is because my lab works on these things. So now you know you are in trouble. Here is a picture of the simplest of reaction centers:
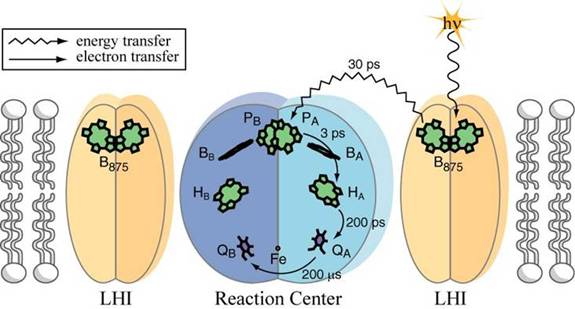
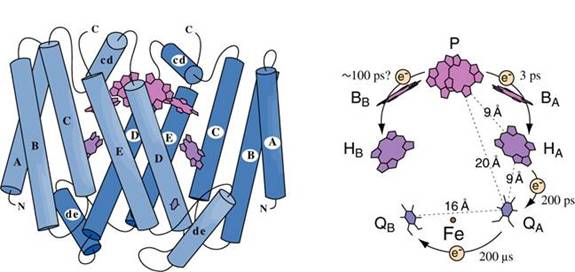
I am not going to go into this too much, but the cofactor P is a pair of bacteriochlorophyll molecules, BA is a single bacteriochlorophyll molecule, HA is a bacteriopheophytin molecule and QA and QB are quinones. We are not going to worry about the other things. When light is absorbed by P it forms an excited state P*. This state transfers an electron presumably through BA and then to HA and then QA and then QB.
P à P* (light energy absorbed, 1.4 eV)
P* à P+ + e- (E0
= +.505 V)
BA + e-
à BA-
HA + e-
à HA-
QA + e- à QA-
QB + H+ + e- à HQB
HQB + H+ + e- à H2QB
The last couple of reactions are rather oversimplified (the order of electron transfer and proton uptake is not quite that shown), but they are conceptually correct.
In class, we will go through the following:
We will write out the full reactions that happen (e.g., P* BA à P+ BA-)
We will decide how we could determine the reaction potentials from the potentials of the half reactions.
We will then use our knowledge of equilibrium to design an experiment to determine the energetics of each reaction.
From this overall energetics of each reaction we will determine the oxidation potentials of all the components in the reaction center.