For a spontaneous reaction:
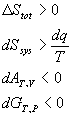
For a reversible reaction:
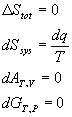
For an isothermal ideal gas:
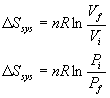
In general:
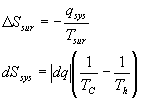
At const. Pressure
![]()
At const. Volume
![]()
Carnot Engine:
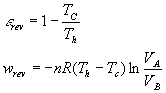
For a transition:
![]()
For chemical reactions:
![]()
In general:
A=U-TS
G=H-TS
Const. Pressure:
dG=dH-TdS
Const. Volume
dA=dU-TdS
In General:
dU=TdS - PdV
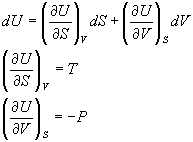
dG = VdP - SdT
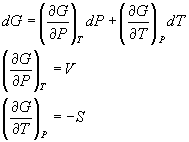
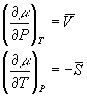
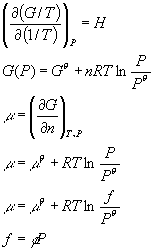
Useful Constants For Water --
CS (specific heat capacity of ice) = 1.95 J/( K g)
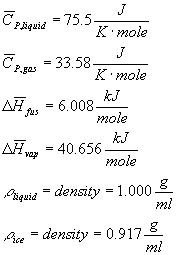 Boiling point = 100
C
Boiling point = 100
C
Freezing point = 0 C
Molecular weight = 18
For a monoatomic ideal gas:
CV = 3R/2
CP = 5R/2