Acid/Base
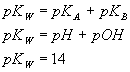
Titrations (weak acid titrated with strong base). Given in terms of activities:
Start point: ![]()
Near the half stochiometry point: ![]()
At the stochiometry point: ![]()
Past the stochiometry point: ![]()
Activity coef.
for monovalent salt:
![]()
For Mn+ Xm- :
![]()
![]()
![]()
for an aqueous solution:
![]()
Electrochemistry
D
rG = -nFE![]()
F = 9.65 x 104 C/mole
solubility :
MX ß à M+(aq) + X-(aq)
KS = aM+ aX-
![]()
Chemical dynamics:
![]()
First order:
![]()
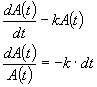
![]()
second order:
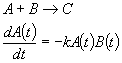
Equilibrium approximation
A ß à B à C
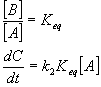
Steady state approximation
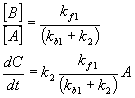
Reaction rate vs. temperature and EA.
![]()