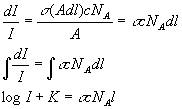
Consider a cuvette with a 1 cm2 area and a pathlength of 1 cm. l is the length that the light has traveled through the cuvette, A is the area of the cuvette, c is the concentration of the molecule in question, s is the optical cross section of the molecule (see below) and NA is avagodro's number. Optical cross section is the effective size of the molecule for absorption of a particular wavelength of light. That is, if we think of the molecule as a black circle absorbing everything that hits it, what effective size would it be for the amount of absorbance it has at any particular wavelength of light? Let's think about the first thin sheet of liquid in the front face of the cuvette that has an area of A and a depth of dl. The fraction of the light absorbed by all the molecules in a thin sheet of molecules is just the effective area of all the molecules in the thin sheet divided by the total area of the thin sheet. The effective area of the molecules in that thin sheet is the number of molecules in the sheet times the optical cross section of each molecule. The number of molecules in the sheet is the volume of the sheet times the concentration of the molecules. The volume of the thin sheet is just A times dl. Putting all this together we have:
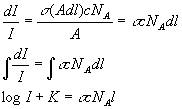
We know that when c = 0, the full intensity of light passes through the cuvette (I0):
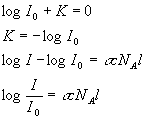
Now, since sNA is just another constant which depends on the wavelength of light and the particular properties of the molecule, we give this a new name. We call it the extinction coefficient, e.
![]()