Research Interests
Current Projects:
Gradient Dielectrophoresis: (Bioanalysis, Proteomics, Biomarkers)
In recent years, dielectrophoresis has emerged as a unique and useful tool for manipulating and capturing small particles. Dielectrophoretic force arises when a non-uniform electric field acts upon permanent or induced dipoles. Insulator-based dielectrophoresis (iDEP) involves the use of insulating geometric features within a microchannel to shape an applied electric field, thus harnessing dielectrophoretic and electrokinetic forces to capture and concentrate particles of interest. Using DEP, seemingly similar cells can be differentiated based on subtle distinctions such as antigen type on erythrocytes, or not-so-subtle differences like living versus dead bacteria. We use microchannels with a graduated, repeating pattern to selectively capture bioparticles from a complex mixture.
PublicationsBiophysical Separation of Staphylococcus epidermidis Strains Based on Antibiotic Resistance. Paul V. Jones, Shannon Huey, Paige Davis, Ryan McLemore, Alex McLaren, Ryan Yanashima, and Mark A. Hayes*Analyst, 2015, Published online. DOI: 10.1039/C5AN00906E. article
Development of the Resolution Theory for Gradient insulator-based Dielectrophoresis. Mark A. Hayes & Paul V. Jones Electrophoresis 2015, 36(9-10), 1098-1106 pub online 5-5-2015, DOI: 10.1002/elps.201400504. article
Differentiation of Escherichia coli Serotypes Using DC Gradient Insulator Dielectrophoresis. Paul V. Jones, Alexa F. DeMichele, LaKeta Kemp, and Mark A. Hayes Anal. Bioanal. 2014, 406(1), 183-192 DOI: 10.1007/s00216-013-7437-5. article
Sarah J. R. Staton, Paul V. Jones, Ginger Ku, S. Douglass Gilman, Indu Kheterpal, and Mark A. Hayes* Manipulation and Capture of Aß Amyloid Fibrils and Monomers by DC Insulator Gradient Dielectrophoresis (DC-iGDEP), Analyst, 2012, 137(14), 3227-3229 DOI:10.1039/C2AN35138B. article
Paul V. Jones, Sarah J. R. Staton, and Mark A. Hayes* Blood cell capture in a gradient dielectrophoretic microchannel. Anal. Bioanal. 2011, 401, 2103-2111, PMID: 21830138.article
Staton, S.J.R., Chen, K.P., Taylor, T.J., Pachecho, J.R., and M.A. Hayes. Characterization of particle capture in sawtooth patterned insulating electrokinetic microfluidic device. Electrophoresis. 2010, 31, 3634-3641.
Chen, K.P., Pacheco, J.R., Hayes, M.A., and S.J.R. Staton. Insulator-based dielectrophoretic separation of small particles in a sawtooth channel. Electrophoresis. 2009, 30, 1441-1448.
Pysher, M.D. and M.A. Hayes. Electrophoretic and dielectrophoretic field gradient technique for separating bioparticles. Analytical Chemistry. 2007, 79, 4552-4557.
Future Directions: Apply to full range of bioparticulates. Current work is blood cells and proteins, amyloid fibrils, virus particles, bioaerosols, and bacteria. Selective capture of 200 nanometer polystyrene particles while excluding 1 micron particles by DC-iGDEP (Staton 2010 - Electrophoresis)
Protein aggregate capture of amyloid A-beta (1-40) fibrils. Voltage removed at 2 s, reapplied at 19 s. (Staton 2012, The Analyst)
Electrophoretic Capture/Separations-Based Arrays: (Bioanalysis, Proteomics, Bioaerosols)
Electrokinetic separations juxtaposed by flow fields represent a valuable tool for separating complex mixtures. A novel separations technique that can dynamically capture specific species in bulk solution from a complex mixture without molecular recognition elements is presented here. The basic premise of the new device is based on electrophoretic principles and exploits differential transport near the capillary entrance by employing a large contraction ratio and setting flow and electric field gradients opposite one another.
PublicationsMeighan, M., Vasquez, L., Dziubcynski, L., Hews, S., and M.A.Hayes. Investigation of Electrophoretic Exclusion Method for the Concentration and Differentiation of Proteins. Analytical Chemistry. 2011, 83, 368-373. article
Meighan, M., Keebaugh, M., Quihuis, A., Kenyon, S., and M.A. Hayes. Development of an Electrophoretic Exclusion Technique for the Selective Transport of Small Molecules. Electrophoresis, 2009, 30, 3786.
N. A. Polson, D. P. Savin, M. A. Hayes. Electrophoretic Focusing Preconcentration Technique in Continuous Buffer Systems Employing Capillary Electrophoresis Separation Systems. Journal of Microcolumn Separations. 2000, 12, 98-106.
Future Directions: Array-based separations. High Sensitivity Micro-Immunoassay Detection: (Quantitative Bioanalysis)
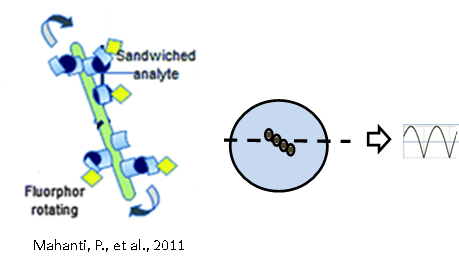
Immunoassays provide a valuable detection and quantification method for biological samples. This work focuses on a novel detection platform utilizing streptavidin-coated silane particles possessing an iron oxide core as a solid capture surface. The introduction of a magnetic field causes supraparticle linear structures to form, and field manipulation produces a periodic change fluorescent signal intensity that can be exploited by signal processing strategies and analyzed independently from unbound analyte.
Publications
Christine F. Woolley and Mark A. Hayes*, Recent Developments in Emerging Microimmunoassays, Bioanalysis, 2013, 5, 245-264.
Prasun Mahanti*, Thomas Taylor*, Mark A. Hayes*, Douglas Cochran, and Matthew M. Petkus. Improved detectability and signal strength for rotating phase fluorescence immunoassays through image processing, Analyst, 2011, 136, 365-373.
Mark A. Hayes*, Matthew M. Petkus, Antonio A. Garcia, Tom Taylor, and Prasun Mahanti. Demonstration of sandwich and competitive modulated supraparticle fluoroimmunoassay applied to cardiac protein biomarker myoglobin, Analyst, 2009, 134, 533-541.
Matthew M. Petkus, Melissa McLauchlin, Anil K. Vuppu, Lynnette Rios, Antonio A. Garcia, and Mark A. Hayes*. Detection of FITC-cortisol via Modulated Supraparticle Lighthouses, Anal. Chem. 2006, 78, 1405-1411.Future Directions: Incorporate as a detection method for a biomarker panel on a next-generation separations-based microfluidic device.
Illustration of immunoassay rotors and signal processing. (Mahanti 2011, The Analyst)
Capillary Isoelectric Focussing (serum amyloid P, wt1 protein, Protein Variants)
We are working on developing a high resolution separations platform capable of distinguishing between subtle variations of proteins. The most significant part of this development includes coupling a mass spectrometer as a detector for capillary isoelectric focusing. We are exploring how this interface works and what are the capabilities of this technique.
Publications
Weiss, N.G., Nelson, R.W., and Hayes, M.A. Examining Serum Amyloid P Component Microheterogeneity Using cIEF and MALDI-MS. Proteomics, 2011, 11, 106-113.
Weiss, N.G., Zwick, N., and Hayes, M.A. Capillary Isoelectric Focusing Coupled Offline to MALDI-MS. Journal of Chromatography A, 2010, 1217, 179-182.
Timothy Crowley, M. A. Hayes* Analysis of Human Blood Serum Using the Off-Line Coupling of Capillary Isoelectric Focusing to Matrix Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry. Proteomics, 2005, 5(14), 3798-3804.
Future Directions: Unique induced field separations in shaped droplets, direct detection in droplets on hand-held device. Liposomes in Electric Fields (Electrophoresis, BioNanotubules)
The biomimetic nature of liposomes as well as their relative ease of preparation have made them ideal for a variety of application in areas such as pharmaceuticals, cosmetics, gene therapy, and bioengineering. Electric field based techniques continue to be essential for the analysis and separation of bioparticles including lipid vesicles. Complex biological and biomimetic systems exhibit a wide range of intrinsic and field-induced properties; as a result, their electrokinetic behaviors are poorly understood and predicted. Understanding and characterizing these behaviors is crucial for the development of technology involving bioparticles and electric fields. Additionally, a better understanding of electric-field induced deformation can give a significant insight of the nature of similar effects in biological environments. Our work has included the study of the electrophoretic migration of various liposome preparations using capillary electrophoresis (CE), the assessment of theoretical models describing electric field-induced migration of vesicles, and qualitative and quantitative descriptions of liposome deformation caused by electric fields. Fluorescence and bright field microscopy as well as scanning electron microscopy (SEM) have been used to demonstrate the many unique shape changes experienced by vesicles undergoing electrophoresis.
Publications
Castillo, J,A.; Narciso, D.M.; Hayes, M.A. “Bionanotubule Formation from Surface Attached Liposomes using Electric Fields” Langmuir 25(1):391-6, Jan 2009.
Castillo, J.A.; Hayes, M.A. “Bionanotubules from Liposomes.” Methods in Enzymology: Liposomes Part G (465), Dec 2009.
Hayes, M.A.; Pysher M.D.; Chen, K.P. “Liposomes form nanotubules and long range networks in the presence of electric field” J. Nanosci. and Nanotechnol. 7 2283-2286, Jul 2007.
Pysher, M.D.; Hayes, M.A. “Effects of deformability, uneven surface charge distributions, and multipole moments on biocolloid electrophoretic migration” Langmuir 21 3572-3577, Apr 2005.
Pysher M.D.; Hayes, M.A. “Examination of the electrophoretic behavior of liposomes” Langmuir 20 4369-4375, May 2004.
Phayre, A.N.; Farfano, H.M.V.; Hayes, M.A. “Effects of pH gradients on liposomal charge states examined by capillary electrophoresis” Langmuir 18 6499-6503, Aug 2002.Future Directions: Future directions of this project include continuing the enhancement of electric field-based separation of liposomes as well as membrane-associated proteins in a native-like environment by incorporating them into lipid vesicles. We are also interested on further exploring the electric field-induce behavior of other biological systems and networks. Bioaerosols
A significant fraction of atmospheric aerosol particles are either living (microorganisms) or of biological origin (dander, plant and insect debris, etc.). Humans, animals and plants constantly emit microscopic particles of cellular material and protein that can account for up to 25% of atmospheric aerosols. Target bioaerosol particles can be considered information-rich packets that carry biochemical information specific to the living organisms present in the collection settings. Our group is interested in determining the feasibility of bioaerosol fingerprinting based on current understanding of cellular debris in aerosol and arguments regarding sampling, sensitivity, separation and detection schemes.
Our current experimental work has focused on preliminary studies involving the collection and examination of bioaerosol samples from various indoor and outdoor (local and international) environments in order to relate the information obtained from the sample analysis with the corresponding collection setting
Publications
Staton, S.J.R.; Castillo, J.A.; Taylor, T.; Herckes, P.; Hayes, M.A. “Detecting a ‘Naked’ Person in the Desert: Identifying Environmental and Organism Patterns from Bioaerosol Material Using HPLC.” In Preparation.
Castillo, J.A.; Staton, S.J.R.; Taylor, T.; Herckes, P.; Hayes, M.A. “Exploring the Feasibility of Bioaerosol Analysis as a Novel Biochemical Fingerprinting Technology.” In Preparation
Future Directions: Identifying and Integrating appropriate sampling, separation technologies as well as pattern identification and statistical methods to maximize the information that can be obtained from the environments of bioaerosol collection. Superhydrophobic Surfaces
Here we explore how superhydrophobic materials can be used to provide new analytical capabilities. Such materials confine water droplets allowing for unique manipulations to be performed. Previously, we have characterized droplet properties, improved performance of MALDI mass spectrometry, performed isoelectric focusing within a drop, and explored the dynamics of drop cutting.
Cutting a drop with superhydrophobic knife
Nearly cutting a drop with superhydrophobic knife
Publications
Cutting a drop of water pinned by wire loops using a superhydrophobic surface and knife. Ryan Yanashima, Antonio A. García, James Aldridge, Noah Weiss, Mark A. Hayes, James H. Andrews PLoS ONE, 2012, 7(9): e45893. doi:10.1371/journal.pone.0045893. article
Weiss, N.G., Hayes, M.A., Garcia A.A, and R.R. Ansari. Isoelectric Focusing in a Drop. Langmuir, 2011, 27, 494-498. article
McLauchlin, M.L., Yang, D.Q., Aella, P., Garcia, A.A., Picraux, S.T., and M.A. Hayes. Evaporative Properties and Pinning Strength of Laser-Ablated Hydrophillic Sites on Lotus-leaf-like Nanostructured Surfaces. Langmuir 2007, 23, 481-487.
Future Directions: For the future we are working to improve the MALDI MS signal using superhydrophobic surfaces. Also see Antonio Garcia's (ASU) website.