Glycan binding proteins

Antiviral lectins:
a novel concept in anti-HIV therapy
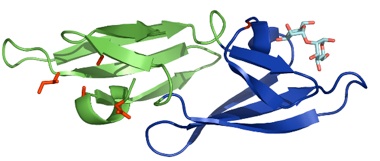
The ability of attaching sugars to proteins, called glycosylation, is typical of eukaryotic cells. However, HIV and related viruses are able to hijack the host’s cellular enzymes to glycosylate their own surface proteins in an attempt to escape the host’s immune response to infection and facilitate viral transmission. These glycans are the target of antiviral lectins, which act as entry inhibitors. Our work focuses on a potent anti-HIV protein, cyanovirin, which binds to oligosaccharides on the viral surface envelope protein gp120. The protein contains two carbohydrate-binding domains, A and B, each of which can bind short oligomannosides in vitro. Work in our lab demonstrated that multivalent interactions are necessary for potent antiviral activity. We are currently using directed evolution and protein engineering to design more potent antiviral lectins based on cyanovirin by optimizing the oligomannose binding site to further improve its affinity, and by reengineering the protein to better control its oligomerization state.
this work is in collaboration with Prof. S. Banu Ozkan (Physics, ASU) and Prof. Claudio J. Margulis (University of Iowa)