Ammonium Ion, NH4+
General Description and Properties:
Ammonium ion is formed by the reaction between acids and aqueous ammonia:
The ammonium ion behaves chemically like the ions of the alkali metals, particularly potassium ion, which is almost the same size. All ammonium salts are white and soluble.
Characteristic reactions of NH4+:
Sodium Hydroxide:
Addition of concentrated hydroxide ion solutions evolves NH3 gas:

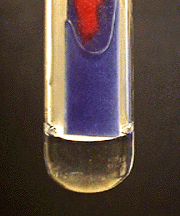
The ammonia gas turns moistened red litmus paper blue. It can also be detected by its characteristic odor. This reaction serves as a confirmatory test for NH4+.
No Reaction:
Cl-, SO42-, NH3(aq)