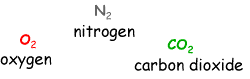
States of Matter and Chemical Reactions
The main ideas we are trying to get across in this presentation are visual demonstrations of the states of matter and observations of mixtures and chemical change. Other ideas and concepts that are touched on include observation and recording methods, measures of accuracy, simple math facts/geometrical shapes, chemical/scientific notation and language.
The kids will all have a copy of THIS assignment sheet.
Introduce Yourself!
Tell the kids your name(s), what you are studying, why you are studying. Why you wanted to go to college, what you plan to do when you leave college, why you like college. Try to minimize questions about college life at this point (this shouldn't be difficult, they will probably be shy at the start), and return to this at the end.
Next the soap bubbles.
Ask the kids what they are surrounded by, i.e the atmosphere.
Is it solid, liquid or gas? How do they know it is there, can they see it?
Ask them to blow bubbles. Can they see the air now (still no, but they can see where the air is)?
Get the kids to answer the state-of-matter questions about the bubbles, soap solution and the plastic container.
What gasses are in air? Write the chemical structures and names of oxygen, nitrogen and carbon dioxide on the board (as below).
Why do chemists confuse things by using the chemical structure symbols, why aren't the simple names good enough?
Leave this question hanging, you will answer it later.
Now for the first demo, Elephant's Toothpaste.
Tell them you are going to use the chemical hydrogen peroxide. Ask them if they have heard of it, do thay have any at home, when do they use it? If they put it on a cut, does it hurt, does anything else happen? Encourage them to think about the fact that it can fizz aswell as hurt on a cut!.
Show them the measuring cylinders. Ask a kid to come up and look at the markings on the cylinder. Help them answer the questions on the assignment sheet (if necessary).
The procedure for the demo is as follows:
1. Add 50 mL yellow washing-up liquid dye to a 50 mL Measuring Cylinder. Ask a kid to read the volume.
2. Pour it into the 1L Measuring Cylinder. Ask a kid to recheck the volume. Is it the same? If not, why not? (introduce the idea of experimental error)
3. Add 50 mL 30% hydrogen peroxide to a different 50 mL Measuring Cylinder. (someone wearing gloves whould do this). Ask a kid to read the volume.
4. Pour into the same 1L Measuring Cylinder and swirl to mix the solutions. Ask a kid to read the total volume. Do the volumes add up correctly. If not, then why not?
5. Ask the kids to decide what happened when these were mixed, was there a change in color, temperature, state of matter, chemical reaction? Ask a kid to come and test the temperature.
6. Add 10 mL 3M potassium iodide (KI) solution to a 50 mL Measuring Cylinder. Ask a kid to read the volume.
7. Ask a kid to get ready to check the new total volume in the 1L Measuring Cylinder after you add the KI solution (but make sure he/she stays in their seat!).
8. Pour the KI solution into the 1L Measuring Cylinder (someone wearing goggles should do this). Tell the kid he/she won't be needed to check the total volume!
9. Ask the kids if anything happened :) Was there a change in color, temperature, state of matter, chemical reaction? If the cylinder is warm, ask a kid to come and touch only a part that doesn't have foam over it (we don't want any of them getting hydrogen peroxide or iodine/iodide on their hands).
10. Do the whole thing again just for fun with red food dye this time (needs a LOT of red food dye). You can tell them that red and yellow are the colors of ASU. Ask the kids how much of the various liquids to use. Tell them to refer to their notes.
Help them interpret what happened. Don't give them the answers to the assignment questions, nudge them in the right direction as appropriate. The idea is to make them think, give them, plenty of time to do this.
The first mixture produced just that, simply a mixture.
The second produced a chemical reaction (there was a change in state of matter and temperature).
A gas was formed, oxygen, that made bubbles in soap just like they did, there were just a lot more of them!
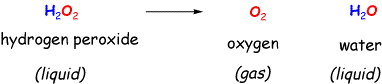
Draw the structure of two hydrogen peroxide molecules on the board and write the name (below). Draw an oxygen molecule on the board and write the name. Point out that the structures show how the peroxide and the oxygen are related (no details here). Tell them that the hydrogen peroxide you used is just like they have at home, only stronger!
Ask what happened to the H's on the hydrogen peroxide. Draw the structure of water and the name. Show that the names don't really say what happened, but the structures do, e.g. they say where the oxygen came from and that the remainder of the hydrogen peroxide molecule became water. Obviously you should talk in generalities here, no details, and you don't have to balance the equation :) An important point not to be missed, however, is that the liquid hydrogen peroxide change states into the gaseous oxygen. This is an obvious indication of a chemical reaction!
Now for the second demo, Genie in the Bottle. The procedure is as follows:
1. Tell the kids that you are going to do some magic, then some science.
2. The Genie bottle is pre-prepared with 30 mL 30% hydrogen peroxide and a small spatula end-full of manganese dioxide powder in a tissue trapped at the neck by the stopper. Tell the kids to watch what happens when you remove the stopper. (someone wearing goggles should do this)
3. Ask what they think happened (was it just a mixture or a chemical change etc). A gas was clearly formed, oxygen again.
4. Tell them that scientists don't do magic they explain and understand things like this. Add 20 mL of the 35% hydrogen peroxide to a 250 mL Measuring cylinder (not used previously). (again, someone wearing gloves and goggles should do this)Ask the kids to watch and then add a small spatula end-full of manganese dioxide. (again someone wearing goggles should do this)
5. Check the temperature of the Measuring cylinder and when cool enough (don't waut until it cools too much!) ask a kid to come and check the temperature.
Again, help them interpret what happened. Help them to understand that the same chemical reaction took place, but just a lot faster.
Ask them what would have happened if soap had been added this time!
Help them answer the questions on the assignment sheet (but don't help too much!)
Depending upon how much time there is left encourage the kids to ask questions about the demos, college life etc.
Direct the presentation in whatever direction seems most appropriate for your particular class and situation.