Conceptual Problems (each problem is worth 6 points). With the exception of problem 1,
you must give a brief explanation of your answer for full credit.
- The second law states that
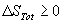 for any process
that can occur. Write a similar inequality for DG at constant
pressure and temperature.
for any process
that can occur. Write a similar inequality for DG at constant
pressure and temperature.
Answer
- Consider a spontaneous, endothermic reaction occurring isothermally in a liquid at
constant pressure as shown in the illustration. Is the entropy change in the bath
(the surroundings) positive, negative, zero or impossible to determine? Explain.
Answer
- For the endothermic reaction in question 2, is the entropy change in the system
positive, negative, zero or impossible to determine? Explain.
Answer
- For the endothermic reaction in question 2, is the Gibbs free energy change
positive, negative, zero or impossible to determine? Explain.
Answer
- At 1 atm and 25 C, is the chemical potential of liquid water greater than, equal to, or
less than the chemical potential of ice (assuming the same amount of both)? How did you
arrive at this conclusion?
Answer
Numerical Problems. Note, express all energies in joules or kilojoules and all
entropy changes in joules per liter.
- (10 points)
0.1 mole of a monoatomic ideal gas in an elastic balloon at 298 K is
heated by 50 K and expands from 1 liter to 2 liters in the process. Calculate the change
in entropy of the system.
Answer
- (15 points)
Determine the entropy change in the system, the entropy change in the
surroundings and the change in Gibbs free energy for the isothermal expansion of 1 mole of
an ideal gas from 1 liter to 10 liters at 298 K against an external pressure of 1 atm.
Answer
- (25 points)
20 kJ of heat is transferred to 100 grams of ice initially at -20 C at
atmospheric pressure (pressure is held constant). a) Determine the final temperature. b)
Determine the amount of liquid water (in mls) and solid ice (in grams) present at the
final temperature. c) Determine the entropy change (in the system) for the process.
Answer
Applied Problem (20 points)
- a) You are an engineer and your company is considering building electrical generators
out in floating stations on the ocean by using something like a Carnot engine. Your job is
to figure out how well this might work. The water at the top of the ocean would serve as
the source of heat and the water at the bottom of the ocean would serve as the cold sink.
Let's say that the top of the ocean was at 15 C and the bottom was at 1 C. Assume that the
system works with no losses other than those required by the second law of thermodynamics
(everything is reversible and the conversion of mechanical to electrical energy is
perfect). How many kilowatts of power could be produced assuming that you could transfer
heat from the heat source (the water near the surface) into the Carnot engine at a rate of
one million joules per second? A kilowatt is a thousand joules per second.
b) Now you are the environmental analyst working with the company, and you foresee a
potential problem with doing this on a large scale. There might be a substantial change in
the water temperature both at the surface and at the bottom of the ocean after a while,
disturbing the sea life. How many liters of surface water could be cooled by one degree
each day if this engine ran all the time?
Answer