Physical and Chemical Properties of Polymer Materials
The main ideas we are trying to get across in this presentation are that everything is made of molecules, that molecules are made of atoms, that molecules are really really small, and that different molecular structures result in different physical properties. We have chosen to use polymeric materials for a variety of reasons. This demo, together with the corresponding Pre-Demo Assignment, integrates elements of the Arizona Department of Education Science Standards.
The kids will all have a copy of THIS assignment sheet.
Introduce Yourselves!
Write your name(s), major(s) and career aspiration(s) on the board.
Get them to answer the questions about you on their assignment sheet.
Introduce the Idea of Molecules
Remind the kids that they recently looked at some polymer materials (they studied silly putty, play dough, gak etc.) and studied their physical properties (bounce, stickyness, stretch etc.). If they didn't realize it, explain that these toys are examples of polymers.
Tell them that today they are going to look at some more polymer materials. The difference this time is that they are going to make the materials themselves, and that they are going to look at them the way that a chemist does.
Tell them that a chemist thinks about materials in terms of molecules. Everything is made of molecules, a chemist tries to understand why materials are the way that they are by thinking about the different molecules that make up different materials.
Pick up a piece of silly putty and tell them that the silly putty is made of many many silly putty molecules. If we wanted to look at a molecule, we could try to separate it from all of the other molecules.
Get a helper to cut the piece of putty into two pieces on an overhead projector, so that the kids can see (the size and shape) of the pieces. Tell your helper that the cut piece still contains many molecules and to cut it again (into 2 pieces). Same thing again, it contains too many molecules, keep cutting (again into 2). Repeat until your helper complains that he/she can't cut it any more, and the piece is just visible on the overhead.
Tell them that even this tiny piece still contains many many molecules, and get them to estimate how many.
Get them to record their estimate on their assignment sheet.
Tell them that a scientist would estimate that the piece would contain about 10^16 molecules. Write this number on the board with ALL of the zero, then write it again "as a scientist would", i.e. in exponent format. They may not exactly understand the number in this format, but they do know that numbers like this are used by scientists. One of the big issues they have been dealing with in 5th grade is scale and orders of magnitude, so they will appreciate what the large number means.
Get them to record your estimate on their assignment sheet, in both numeric formats.
Ask them how big they think a molecule must be if so many are in a piece so small. Don't get technical here, just say emphasize the fact that a molecule is so small that nobody could hope to see it. Scientists can get a good idea of what molecules look like, however, and you are going to show them a picture of a Silly Putty molecule, in fact this one, on the overhead (transparency provided).
Ask them to look closely at the picture of the molecule, get them to identify the atoms. Tell them that all molecules are made of atoms.
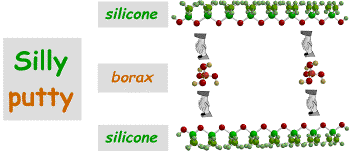
At this point you can say that Silly Putty actually contains 2 molecules that are joined together (the hands). The important point here, however, is to point to the atoms in the molecule. Ask if anybody knows what they are. They may or may not know the answer to this one. Tell them that they are atoms
Get them to answer the questions about molecules, size and atoms on their assignment sheets.
Now to the Fun Part, Making Polymers
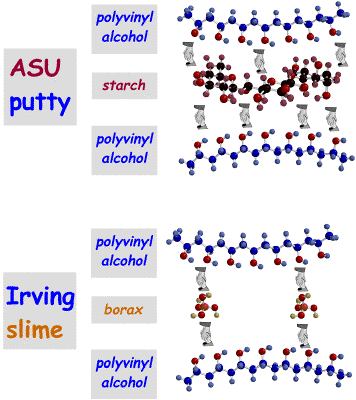
Tell them that they are going to make their own polymer materials that we call Irving Slime and ASU Putty. Show them the pictures of these molecules on the overhead.
Before they start, you must get them to pay attention to the Rules. Put the Rules transparency on the overhead, and make sure that they listen carefully! Tell them that their hands will get colored, but won't be harmed by the color. If they like they can wear gloves, because they will not have time to wash their hands!

The kids will be divided into five teams of around 4-6 kids, each team has a kit. Each kit contains instructions to make 6 polymers. Each kit has a glue bottle labelled A1, A2, A3, and a water/glue bottle labelled A4, A5, A6. Each also has three starch solutions, B1, B2, B3 and also three Borax solutions, B4, B5, B6. The instructions tell the kids how to mix the polymers. The instructions are pretty clear. Only help the kids if they look like they really need it. They will look for help, but if you don't offer it at first they will have to read the instructions, which is what we want them to do. Although there are various colors, there are only two polymers, a slimy one we call Irving Slime and a more solid one we call ASU Putty.
Then to make the polymers.....
We use the following instruction sheets for the various polymers, Polymer 1, Polymer 2, Polymer 3, Polymer 4, Polymer 5, Polymer 6.
The main problem the kids will have is that they will not stir the polymers enough and end with something that is still wet and slimey. Encourage them to stir (putty) and swirl (slime) their polymers for a long time. After a while they can get their fingers into them! This really helps, rolling and stretching will make good slime and putty material.
This part can last as long as you like, the kids will be having fun, but you will need to allow 15 minutes at the end for wrap-up. Get the kids to put all of their polymers in zip-lock bags, and they MUST put them away in their desks and not be allowed to play with them anymore, or they will not pay attention. Tell them to return everything to the kit boxes and toss everything that has polymer on it into garbage bags.
Get the kids to answer the questions about the chemicals, reactions and polymers on their assignment sheets. Explain that a chemist calls the "hand-holding" forming a chemical bond.
Irving Student Questions
End by telling them that this demo was inspired by some good questions that Irving students asked when they visited MCC. In a group, have them answer the final three questions on the assignment sheet.
The final thing is to ask if they have any questions about the demos, polymers, molecules, atoms or college life. This part you will have to play by ear. If you finish early, you might want to tell what you are studying, about campus life etc.

Arizona Science Standards Items Addressed in this Demo
ESSENTIALS (Grades 4-8)
5SC-E1. Examine, describe, compare, measure, and classify objects and mixtures of substances based on
common physical and chemical properties.
(Grades 4-5)
PO 1. Identify common physical and chemical properties.
PO 2. Compare physical and chemical properties of common objects.
PO 3. Compare physical and chemical properties of common mixtures
(Grades 6-8)
PO 1. Classify objects and mixtures of substances based on physical and chemical properties.
PO 2. Analyze physical and chemical properties of objects and mixtures
5SC-E2. Classify and describe matter in terms of elements, compounds, mixtures, atoms and molecules.
(Grades 4-5)
PO 1. Distinguish among matter, mixtures and compounds.
(Grades 6-8)
PO 1. Classify matter in terms of elements, compounds, mixtures, atoms and molecules.
PO 2. Describe elements, compounds, mixtures, atoms and molecules as they relate to matter.